Abstract
Emerging evidence suggests that aberrant O-GlcNAcylation is associated with tumorigenesis. Many oncogenic factors are O-GlcNAcylated, which modulates their functions. However, it remains unclear how O-GlcNAcylation and O-GlcNAc cycling enzymes, O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), affect the development of cancer in animal models. In this study, we show that reduced level of OGA attenuates colorectal tumorigenesis induced by Adenomatous polyposis coli (Apc) mutation. The levels of O-GlcNAcylation and O-GlcNAc cycling enzymes were simultaneously upregulated in intestinal adenomas from mice, and in human patients. In two independent microarray data sets, the expression of OGA and OGT was significantly associated with poor cancer-specific survival of colorectal cancer (CRC) patients. In addition, OGA heterozygosity, which results in increased levels of O-GlcNAcylation, attenuated intestinal tumor formation in the Apcmin/+ background. Apcmin/+ OGA+/− mice exhibited a significantly increased survival rate compared with Apcmin/+ mice. Consistent with this, Apcmin/+ OGA+/− mice expressed lower levels of Wnt target genes than Apcmin/+. However, the knockout of OGA did not affect Wnt/β-catenin signaling. Overall, these findings suggest that OGA is crucial for tumor growth in CRC independently of Wnt/β-catenin signaling.
Similar content being viewed by others
Introduction
O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA) are enzymes that regulate the addition and removal of monosaccharides of O-linked β-N-acetylglucosamine to the Ser and Thr residues (O-GlcNAc) of target proteins, respectively.1 Increasing evidence suggests that OGT and/or OGA expression and O-GlcNAc levels are altered in different types of cancer, including colon, lung,2 breast,3, 4 liver,5 prostate,6, 7 pancreas8 and bladder.9 Furthermore, tumor aggressiveness is closely associated with the levels of O-GlcNAcylation, OGT and/or OGA. Consistent with this, various O-GlcNAc-modified proteins are perturbed in tumorigenesis. For example, O-GlcNAc-modified proteins are involved in cancer-relevant processes such as transcriptional regulation, cell proliferation and the cell cycle.10 The activity of many oncogenic factors, including c-Myc,11 β-catenin,12 p5313 and FoxM1,14 is regulated by direct O-GlcNAcylation. In addition, O-GlcNAc cycling is required for the precise control of the cell cycle, suggesting an essential role for O-GlcNAc cycling enzymes in cell proliferation.12, 15, 16
Colorectal cancer (CRC) is the third common cancer worldwide. Adenomatous polyposis coli (Apc) is a tumor suppressor gene that is mutated in ∼80% of sporadic adenomatous polyps and CRCs.17 Mutant APC causes the oncogenic activation of β-catenin. Notably, β-catenin is directly O-GlcNAcylated. Several studies have suggested that the O-GlcNAcylation of β-catenin affects its transcriptional activity and subcellular localization.12, 18 Moreover, higher levels of O-GlcNAcylation and OGT expression are found in colon tumors than in corresponding non-tumorous mucosal tissues.2 Although current evidence implicates O-GlcNAcylation in CRC, it remains unclear exactly how the levels of O-GlcNAcylation or O-GlcNAc cycling enzymes affect colorectal tumorigenesis. To evaluate the role of OGA in colorectal tumorigenesis, we investigated whether OGA heterozygosity could alter CRC susceptibility in Apcmin/+ mice.
We found that the levels of O-GlcNAcylation and O-GlcNAc cycling enzymes were elevated simultaneously in human and mouse colorectal tumors. Increased expression of the O-GlcNAc enzymes OGT and OGA was correlated significantly with poor survival. Therefore, OGA heterozygosity attenuates colorectal tumorigenesis in Apcmin/+ mice. Although β-catenin is O-GlcNAcylated, elevated levels of O-GlcNAcylation did not affect Wnt/β-catenin signaling. Taken together, our observation supports the hypothesis that OGA plays a key role in intestinal tumorigenesis.
Results and discussion
Increased O-GlcNAcylation and O-GlcNAc cycling enzymes in colorectal adenomas
Previous studies have suggested that abnormal levels of O-GlcNAcylation and O-GlcNAc enzymes are closely linked to tumorigenesis. However, it is unclear whether O-GlcNAcylation plays a role in colorectal tumorigenesis in vivo. First, we analyzed the expression pattern of O-GlcNAcylation and O-GlcNAc cycling enzymes in CRC tissues from Apcmin/+ mice and human patients. Colonic homogenates of normal mucosa and adenomas from Apcmin/+ mice were analyzed by immunoblotting to assess the levels of O-GlcNAcylation and O-GlcNAc cycling enzymes. Interestingly, we observed that O-GlcNAcylation was increased significantly (greater than twofold) in colonic adenomas compared with normal mucosa (Figures 1a and b). Colonic adenomas also expressed higher levels of OGT and OGA than normal mucosa (Figures 1c and d). Consistent findings were observed in human patients with CRC (Figures 1e–h). Surprisingly, both OGT and OGA enzymes were increased simultaneously in colonic adenomas. This suggests that the upregulation of OGA compensates for the increased O-GlcNAcylation. Conversely, we also observed an OGA deletion, which elevates O-GlcNAcylation and downregulates OGT.15 These results suggest that the abnormally elevated levels of O-GlcNAcylation and its cycling enzymes are relevant to colorectal tumorigenesis.
The levels of O-GlcNAc cycling enzymes are elevated in mice and human patients with colonic adenomas. (a–c) The levels of OGA (a), OGT (b) and O-GlcNAc (c) in normal mucosa (N) and Apcmin/+ mouse adenomas (A) were compared by western blotting using the following antibodies: the anti-OGT and -OGA polyclonal antibodies, which had been generated previously and were used as described,25 O-GlcNAc (RL2) (MA1-072; Thermo Fisher Scientific Inc., Waltham, MA, USA) and anti-β-actin (691001; MP Biomedicals, Santa Ana, CA, USA). Densitometry was performed to quantify the immunoblots, and the ratios of OGA, OGT and O-GlcNAc to β-actin were determined. (d–f) The same experiment was performed with CRC samples isolated from human patients. Densitometry was performed on immunoblots as described above. Error bars represent the standard deviation (s.d.; n=3). **P<0.005, *P<0.05 (Student’s t-test). The human specimens were obtained from the Department of Pathology, Yonsei University (Seoul, Korea), and from the Liver Cancer Specimen Bank of the National Research Resource Bank Program of the Korea Science and Engineering Foundation of the Ministry of Science and Technology. Authorization for the use of these tissues for research purposes was obtained from the Institutional Review Board of Yonsei University of College of Medicine (IRB number: 4-2012-0026).
Association of O-GlcNAc cycling enzyme expression with survival and cancer recurrence in patients with CRC
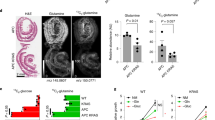
Because the aberrant expression of OGT and OGA has been reported in different types of cancer, we assessed whether OGT and OGA expression exhibits prognostic value in CRC. Two independent cohorts of CRC patients from the Moffitt Cancer Center (GSE17536)19 and a Norwegian hospital (Norwegian; GSE30378)20 were analyzed for the association between OGT and OGA expression and patient survival and cancer recurrence. The Moffitt Cancer Center data set reported the disease-specific survival times for 177 CRC patients. The gene expression levels of OGT and OGA had a strong positive correlation (Pearson’s correlation=0.71; P-value <2.2e−16), supporting the upregulation of these genes in cancer (Figures 1a and e). Expression was also significantly associated with the disease-specific survival probability: OGT and OGA had hazard ratios of 2.26 (P=0.00245) and 2.42 (P=0.0155). Patients with reduced levels of OGT and OGA exhibited improved survival rates (Figures 2a and b).
Association of the expression levels of O-GlcNac cycling enzymes with CRC patient survival and relapse. (a) The MCC cohort was divided into two groups according to O-GlcNac enzyme levels, and the Kaplan–Meier curves of disease-specific survival were compared. (b) The Norwegian patient cohort was divided into two groups according to O-GlcNac enzyme levels, and the Kaplan–Meier curves of relapse-free survival were compared. The split with the lowest P-value in the log-rank test was selected from among the median 60% of samples sorted by gene expression levels. The hazard ratios and P-values shown in the plots were calculated from Cox’s proportional-hazard regression model.
OGA heterozygosity reduces the number of intestinal tumors and increases the survival of Apcmin/+ mice
We next used OGA+/− mice to explore the effect of increased OGT and OGA expression on colorectal tumorigenesis. Apcmin/+ mice were crossed with OGA+/− mice. The intestines of OGA+/− mice showed reduced levels of both OGA and OGT expression compared with control mice (Figure 3a). We demonstrated previously that OGA−/− mice displayed perinatal lethality, and that OGA−/− mouse embryonic fibroblasts downregulate OGT, which might compensate for the increased levels of O-GlcNAcylation.15 Therefore, OGA+/− mice are the correct model for assessing the effect of reduced OGT and OGA expression on colorectal tumorigenesis. A quantitative analysis of tumor burden revealed a significant twofold reduction in the number and size of adenomas in the small intestine of Apcmin/+ OGA+/− compared with Apcmin/+ mice (Figure 3a). Apcmin/+ OGA+/− mice displayed fewer and smaller tumors along the intestinal tract (Figures 3b and c). Apcmin/+ OGA+/− mice exhibited a significantly increased survival rate compared with Apcmin/+ mice, and all Apcmin/+ mice died within 8 months. In contrast, Apcmin/+ OGA+/− mice showed dramatically increased survival rates (Figure 3d).
OGA heterozygosity attenuates APC-mediated intestinal tumorigenesis. (a) The expression of OGA was confirmed in OGA+/− intestines. O-GlcNAc levels were elevated, and OGT was downregulated in OGA+/− intestines. (b) The number (left) and size (right) of intestinal tumors. (c) Gross appearance of the small intestine (upper), and hematoxylin and eosin staining (lower) of intestinal adenoma sections from Apcmin/+ and Apcmin/+ OGA+/− mice at 20 weeks. n=8 mice per group. Error bars represent ±s.e.m. **P<0.005, *P<0.05 (Student’s t-test). (d) Survival rate of Apcmin/+ and Apcmin/+ OGA+/− mice (Kaplan–Meier log-rank, P=0.0001, n=24 and n=15, respectively). Apcmin/+ mice n=24; Apcmin/+ OGA+/− mice n=15. (e) The mRNA levels of Wnt target genes were analyzed using RT2 Profiler PCR Arrays (Qiagen, Valencia, CA, USA). Mice were killed at 8, 16 or 20 weeks and the mRNA levels of (f) Axin2 and (g) Jun were analyzed by qPCR. The primer sequences (mouse) used were: Axin2 forward, 5′-ACTCTGGAGGCTTTCGTTTG-3′; Axin2 reverse, 5′-TTAAGTCAGCAGGGGCTCAT-3′; Jun forward, 5′-CCTTCTACGACGATGCCCTC-3′; Jun reverse, 5′-GGTTCAAGGTCATGCTCTGTTT-3′; GAPDH forward, 5′-AGGTCGGTGTGAACGGATTTG-3′; and GAPDH reverse, 5′-TGTAGACCATGTAGTTGAGGTCA-3′. Error bars represent the s.d. (n=3); **P<0.005, *P<0.05 (Student’s t-test).
Decreased levels of Wnt target genes in Apcmin/+ OGA+/− mice
Based on the attenuated intestine tumor formation in Apcmin/+ OGA+/− mice, we next analyzed molecular changes. We examined whether OGA heterozygosity affects the expression of known Wnt/β-catenin target genes, which are upregulated in APC-mediated intestinal tumors. Nine genes were decreased in Apcmin/+ OGA+/− intestines compared with Apcmin/+ intestines (Figure 3e). We observed that the Wnt target genes Axin2 and Jun were not significantly changed in 8-week-old Apcmin/+ OGA+/− mice compared with age-matched Apcmin/+ controls. However, dramatically reduced expression of Axin2 and Jun was observed in 16- and 24-week-old animals (Figures 3f and g). These results suggest that, although reduced OGA levels do not influence APC-mediated tumor initiation, OGA plays critical roles in tumor growth. Indeed, OGA-deficient cells exhibit a reduced growth rate.15 In addition, OGT knockdown attenuates the growth of breast,14 lung and colon cancer cells.2 These results suggest that reduced OGT and OGA levels disrupt the dynamic regulation of O-GlcNAcylation, which attenuates cell growth.
OGA disruption does not affect Wnt/β-catenin signaling
The activation of β-catenin is essential not only for tumor initiation but also for tumor progression in Apcmin/+ mice.21 Importantly, β-catenin is directly O-GlcNAcylated, and we also observed that β-catenin is modified with O-GlcNAc (Figure 4a). The O-GlcNAcylation of β-catenin regulates its localization and transcriptional activity.22 OGT interacts with β-catenin to regulate cyclin D1 synthesis upon serum stimulation.12 Therefore, we assessed whether OGA deficiency affects Wnt/β-catenin signaling. The deletion of OGA did not affect Wnt3-mediated β-catenin accumulation (Figure 4b). In addition, there were no significant differences in Axin2 and Jun mRNA levels between OGA+/+ and OGA−/− mouse embryonic fibroblasts after stimulation with Wnt3a (Figure 4c). We also used immunoblotting and quantitative PCR to further confirm that the OGA inhibitor Thiamet G and knockdown did not affect Wnt/β-catenin signaling (Figures 4d and e). Increased O-GlcNAcylation after Thiamet G treatment might result in the upregulation OGA to compensate for the increased O-GlcNAcylation. This observation supports the results presented in Figure 3a. These results suggest that OGA heterozygosity affects tumorigenesis independently of Wnt/β-catenin signaling. In addition, intestinal tumorigenesis correlates with OGT and OGA levels, which are important for the dynamic regulation of O-GlcNAcylation.
Elevated O-GlcNAcylation does not affect Wnt/β-catenin signaling. (a) β-Catenin is O-GlcNAcylated. HEK-293 cells were transfected with empty vector or a GFP-β-catenin expression vector. GFP-β-catenin-transfected HEK-293 cells were untreated (−) or treated (+) with Thiamet G. Cell lysates were immunoprecipitated with anti-GFP antibodies and immunoblotting was performed using antibodies against O-GlcNAc (RL-2). (b) Wild-type and OGA+/− MEFs were stimulated with Wnt3a (100 ng/ml) at the indicated time points, and then subjected to immunoblotting. (c) Axin2 and Jun mRNA levels were analyzed by qPCR after Wnt3a treatment for 6 h. (d) Control, OGA knockdown and Thiamet G (#SML0244; Sigma, Madison, WI, USA)-treated HEK-293 cells were stimulated with Wnt3a-conditioned media (CM), and analyzed by western blotting at the indicated time points. (e) The same samples were analyzed by qPCR after 6 h of stimulation. Wnt3a-conditioned media were prepared from mouse L cells (ATCC, Manassas, VA, USA) stably expressing Wnt3a. The primer sequences (human) used were: Axin2 forward, 5′-ATGAGTAGCGCCGTGTTAGTG-3′; Axin2 reverse, 5′-GGGCATAGGTTTGGTGGACT-3′; GAPDH forward, 5′-CCACTCCTCCACCTTTGAC-3′; and GAPDH reverse, 5′-ACCCTGTTGCTGTAGCCA-3′.
Conclusions
Many studies have suggested that elevated O-GlcNAcylation and/or O-GlcNAc cycling enzymes contribute to tumorigenesis. In colon cancer, the levels of O-GlcNAc and OGT are increased.23 However, it remains unclear whether the elevated O-GlcNAcylation caused by increased OGT activity promotes tumorigenesis in vivo. Here, we suggested a critical role for O-GlcNAc cycling enzymes in colorectal tumorigenesis. We observed that human colonic adenomas exhibit elevated O-GlcNAcylation, and increased levels of both OGT and OGA. Interestingly, an analysis of microarray data sets revealed that OGA and OGT expression was correlated. This supports the immunoblotting data, which showed simultaneously increased OGT and OGA in colonic adenomas. Importantly, OGT and OGA are both correlated with survival and cancer recurrence in patients with CRC. Our results demonstrate that OGA heterozygosity reduces APC-mediated colorectal tumorigenesis and enhances the survival of OGA heterozygous mice in the Apcmin/+ background. Wnt target genes were downregulated in Apcmin/+ OGA+/− mice compared with Apcmin/+ controls. Although β-catenin is directly O-GlcNAcylated, elevated O-GlcNAcylation did not affect Wnt/β-catenin signaling in vitro. Although O-GlcNAcylation is elevated in colonic adenomas, we observed reduced tumorigenesis in OGA+/− mice, in which O-GlcNAcylation is constantly elevated. These results suggest that the levels of OGA and OGT are critical for colorectal tumorigenesis. These results support previous studies showing that the knockdown of OGA or OGT reduced cell growth in vitro.14, 15 This suggests that reduced levels of OGT and OGA do not properly regulate dynamic O-GlcNAcylation, which influences the functions of O-GlcNAc target proteins. Nevertheless, the mechanism by which OGA heterozygosity attenuates tumorigenesis remains unclear. However, many studies have suggested that aberrant O-GlcNAcylated proteins contribute to the disruption of cancer cell growth. Notably, many transcription factors, including c-Myc, p53, NF-κB, SP1, FoxM1 and HCF-1, are O-GlcNAcylated.24 O-GlcNAcylation modulates their activity or stability, which affects cancer cell growth. Phosphorylation is regulated by a variety of protein kinases and phosphatases. Although O-GlcNAcylation is similar to phosphorylation, unlike phosphorylation it is regulated by only OGT and OGA, which are encoded by single genes. Therefore, reduced levels of O-GlcNAc cycling enzymes cause various changes in the activity of entire target proteins. Furthermore, reduced levels of OGT and OGA might not normally regulate dynamic O-GlcNAcylation during the cell cycle, which is required for cell cycle control.15, 16 We conclude that O-GlcNAc enzymes are essential for tumor growth in CRC, and that targeting OGA may help suppress the intestinal tumorigenesis initiated by Apc mutation.
References
Hart GW, Housley MP, Slawson C . Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 2007; 446: 1017–1022.
Mi WY, Gu YC, Han CF, Liu HY, Fan QO, Zhang XL et al. O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochim Biophys Acta 2011; 1812: 514–519.
Slawson C, Pidala J, Potter R . Increased N-acetyl-beta-glucosaminidase activity in primary breast carcinomas corresponds to a decrease in N-acetylglucosamine containing proteins. Biochim Biophys Acta 2001; 1537: 147–157.
Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C et al. GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res 2010; 70: 6344–6351.
Zhu Q, Zhou L, Yang Z, Lai M, Xie H, Wu L et al. O-GlcNAcylation plays a role in tumor recurrence of hepatocellular carcinoma following liver transplantation. Med Oncol 2012; 29: 985–993.
Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ . Critical role of O-linked beta-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J Biol Chem 2012; 287: 11070–11081.
Kamigaito T, Okaneya T, Kawakubo M, Shimojo H, Nishizawa O, Nakayama J . Overexpression of O-GlcNAc by prostate cancer cells is significantly associated with poor prognosis of patients. Prostate Cancer Prostatic Dis 2014; 17: 18–22.
Ma ZY, Vocadlo DJ, Vosseller K . Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-kappa B activity in pancreatic cancer cells. J Biol Chem 2013; 288: 15121–15130.
Rozanski W, Krzeslak A, Forma E, Brys M, Blewniewski M, Wozniak P et al. Prediction of bladder cancer based on urinary content of MGEA5 and OGT mRNA level. Clin Lab 2012; 58: 579–583.
Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O . Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem 2011; 80: 825–858.
Itkonen HM, Minner S, Guldvik IJ, Sandmann MJ, Tsourlakis MC, Berge V et al. O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer cells. Cancer Res 2013; 73: 5277–5287.
Olivier-Van Stichelen S, Drougat L, Dehennaut V, El Yazidi-Belkoura I, Guinez C, Mir AM et al. Serum-stimulated cell cycle entry promotes ncOGT synthesis required for cyclin D expression. Oncogenesis 2012; 1: e36.
Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol 2006; 8: 1074–1083.
Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S et al. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene 2010; 29: 2831–2842.
Yang YR, Song M, Lee H, Jeon Y, Choi EJ, Jang HJ et al. O-GlcNAcase is essential for embryonic development and maintenance of genomic stability. Aging Cell 2012; 11: 439–448.
Dehennaut V, Lefebvre T, Sellier C, Leroy Y, Gross B, Walker S et al. O-linked N-acetylglucosaminyltransferase inhibition prevents G2/M transition in Xenopus laevis oocytes. J Biol Chem 2007; 282: 12527–12536.
Fearon ER, Vogelstein B . A genetic model for colorectal tumorigenesis. Cell 1990; 61: 759–767.
Sayat R, Leber B, Grubac V, Wiltshire L, Persad S . O-GlcNAc-glycosylation of beta-catenin regulates its nuclear localization and transcriptional activity. Exp Cell Res 2008; 314: 2774–2787.
Smith JJ, Deane NG, Wu F, Merchant NB, Zhang B, Jiang A et al. Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer. Gastroenterology 2010; 138: 958–968.
Sveen A, Agesen TH, Nesbakken A, Meling GI, Rognum TO, Liestol K et al. ColoGuidePro: a prognostic 7-gene expression signature for stage III colorectal cancer patients. Clin Cancer Res 2012; 18: 6001–6010.
Chan TA, Wang Z, Dang LH, Vogelstein B, Kinzler KW . Targeted inactivation of CTNNB1 reveals unexpected effects of beta-catenin mutation. Proc Natl Acad Sci USA 2002; 99: 8265–8270.
Ha JR, Hao L, Venkateswaran G, Huang YH, Garcia E, Persad S . beta-Catenin is O-GlcNAc glycosylated at Serine 23: implications for beta-catenin's subcellular localization and transactivator function. Exp Cell Res 2014; 321: 153–166.
Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X et al. O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochim Biophys Acta 2011; 1812: 514–519.
Slawson C, Hart GW . O-GlcNAc signalling: implications for cancer cell biology. Nat Rev Cancer 2011; 11: 678–684.
Suh PG, Song M, Kim HS, Parka JM, Kim SH, Kim IH et al. O-GlcNAc transferase is activated by CaMKIV-dependent phosphorylation under potassium chloride-induced depolarization in NG-108-15 cells. Cell Signal 2008; 20: 94–104.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MSIP) (No. 2007-0093861 and No. 2010-0028684) and the Korean Government (MOE) (2013R1A1A2064434).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
Oncogenesis is an open-access journal published by Nature Publishing Group. This work is licensed under a Creative Commons Attribution 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/
About this article
Cite this article
Yang, Y., Jang, HJ., Yoon, S. et al. OGA heterozygosity suppresses intestinal tumorigenesis in Apcmin/+ mice. Oncogenesis 3, e109 (2014). https://doi.org/10.1038/oncsis.2014.24
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/oncsis.2014.24
This article is cited by
-
Thymidylate synthase O-GlcNAcylation: a molecular mechanism of 5-FU sensitization in colorectal cancer
Oncogene (2022)
-
The Dysregulation of OGT/OGA Cycle Mediates Tau and APP Neuropathology in Down Syndrome
Neurotherapeutics (2021)
-
O-GlcNAc transferase associates with the MCM2–7 complex and its silencing destabilizes MCM–MCM interactions
Cellular and Molecular Life Sciences (2018)
-
Memory and synaptic plasticity are impaired by dysregulated hippocampal O-GlcNAcylation
Scientific Reports (2017)