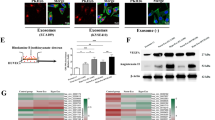
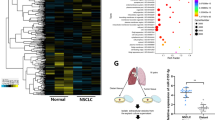
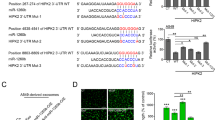
Abstract
Hypoxia plays a critical role during the evolution of malignant cells and tumour microenvironment (TME).Tumour-derived exosomes contain informative microRNAs involved in the interaction of cancer and stromal cells, thus contributing to tissue remodelling of tumour microenvironment. This study aims to clarify how hypoxia affects tumour angiogenesis through exosomes shed from lung cancer cells. Lung cancer cells produce more exosomes under hypoxic conditions than do parental cells under normoxic conditions. miR-23a was significantly upregulated in exosomes from lung cancer under hypoxic conditions. Exosomal miR-23a directly suppressed its target prolyl hydroxylase 1 and 2 (PHD1 and 2), leading to the accumulation of hypoxia-inducible factor-1 α (HIF-1 α) in endothelial cells. Consequently, hypoxic lung cancer cells enhanced angiogenesis by exosomes derived from hypoxic cancer under both normoxic and hypoxic conditions. In addition, exosomal miR-23a also inhibits tight junction protein ZO-1, thereby increasing vascular permeability and cancer transendothelial migration. Inhibition of miR-23a by inhibitor administration decreased angiogenesis and tumour growth in a mouse model. Furthermore, elevated levels of circulating miR-23a are found in the sera of lung cancer patients, and miR-23a levels are positively correlated with proangiogenic activities. Taken together, our study reveals the clinical relevance and prognostic value of cancer-derived exosomal miR-23a under hypoxic conditions, and investigates a unique intercellular communication, mediated by cancer-derived exosomes, which modulates tumour vasculature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A . Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29.
Wu YC, Tang SJ, Sun GH, Sun KH . CXCR7 mediates TGF[beta]1-promoted EMT and tumor-initiating features in lung cancer. Oncogene 2016; 35: 2123–2132.
Quail DF, Joyce JA . Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19: 1423–1437.
Arjaans M, Schröder CP, Oosting SF, Dafni U, Kleibeuker JE, de Vries EGE . VEGF pathway targeting agents, vessel normalization and tumor drug uptake: from bench to bedside. Oncotarget 2016; 7: 21247–22158.
Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014; 25: 501–515.
Schumacher D, Strilic B, Sivaraj Kishor K, Wettschureck N, Offermanns S . Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 2013; 24: 130–137.
Rankin EB, Giaccia AJ . Hypoxic control of metastasis. Science 2016; 352: 175–180.
Hu J, Sun T, Wang H, Chen Z, Wang S, Yuan L et al. MiR-215 is induced post-transcriptionally via HIF-Drosha complex and mediates glioma-initiating cell adaptation to hypoxia by targeting KDM1B. Cancer Cell 2016; 29: 49–60.
Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci USA 2014; 111: E3234–E3242.
Pientka FK, Hu J, Schindler SG, Brix B, Thiel A, Jöhren O et al. Oxygen sensing by the prolyl-4-hydroxylase PHD2 within the nuclear compartment and the influence of compartmentalisation on HIF-1 signalling. J Cell Sci 2012; 125: 5168–5176.
Song D, Li LS, Arsenault PR, Tan Q, Bigham AW, Heaton-Johnson KJ et al. Defective Tibetan PHD2 binding to p23 links high altitude adaption to altered oxygen sensing. J Biol Chem 2014; 289: 14656–14665.
del Peso L, Castellanos MC, Temes E, Martin-Puig S, Cuevas Y, Olmos G et al. The von Hippel Lindau/hypoxia-inducible factor (HIF) pathway regulates the transcription of the HIF-proline hydroxylase genes in response to low oxygen. J Biol Chem 2003; 278: 48690–48695.
Foster JG, Wong SC, Sharp TV . The hypoxic tumor microenvironment: driving the tumorigenesis of non-small-cell lung cancer. Future Oncol 2014; 10: 2659–2674.
Wang SW, Liu SC, Sun HL, Huang TY, Chan CH, Yang CY et al. CCL5/CCR5 axis induces vascular endothelial growth factor-mediated tumor angiogenesis in human osteosarcoma microenvironment. Carcinogenesis 2015; 36: 104–114.
Wang Q, Hu DF, Rui Y, Jiang AB, Liu ZL, Huang LN . Prognosis value of HIF-1α expression in patients with non-small cell lung cancer. Gene 2014; 541: 69–74.
Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife 2016; 5: e10250.
Pefanis E, Wang J, Rothschild G, Lim J, Kazadi D, Sun J et al. RNA exosome-regulated long non-coding RNA transcription controls super-enhancer activity. Cell 2015; 161: 774–789.
Hosseini-Beheshti E, Choi W, Weiswald LB, Kharmate G, Ghaffari M, Roshan-Moniri M et al. Exosomes confer pro-survival signals to alter the phenotype of prostate cells in their surrounding environment. Oncotarget 2016; 7: 14639–14658.
Li L, Li C, Wang S, Wang Z, Jiang J, Wang W et al. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res 2016; 76: 1770–1780.
Sánchez CA, Andahur EI, Valenzuela R, Castellón EA, Fullá JA, Ramos CG et al. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget 2016; 7: 3993–4008.
Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling. Stem Cells 2016; 34: 601–613.
Borges FT, Melo SA, Özdemir BC, Kato N, Revuelta I, Miller CA et al. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol 2013; 24: 385–392.
Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ et al. A microRNA signature of hypoxia. Mol Cell Biol 2007; 27: 1859–1867.
Crosby ME, Kulshreshtha R, Ivan M, Glazer PM . MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res 2009; 69: 1221–1229.
Zhang D, Shi Z, Li M, Mi J . Hypoxia-induced miR-424 decreases tumor sensitivity to chemotherapy by inhibiting apoptosis. Cell Death Dis 2014; 5: e1301.
Ma Y, Yang HZ, Dong BJ, Zou HB, Zhou Y, Kong XM et al. Biphasic regulation of autophagy by miR-96 in prostate cancer cells under hypoxia. Oncotarget 2014; 5: 9169–9182.
Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, Pouysségur J . HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J 2003; 22: 4082–4090.
Trikha P, Sharma N, Pena C, Reyes A, Pécot T, Khurshid S et al. E2f3 in tumor macrophages promotes lung metastasis. Oncogene 2016; 35: 3636–3646.
Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M et al. Nature 2015; 527: 329–335.
Cui H, Seubert B, Stahl E, Dietz H, Reuning U, Moreno-Leon L et al. Tissue inhibitor of metalloproteinases-1 induces a pro-tumourigenic increase of miR-210 in lung adenocarcinoma cells and their exosomes. Oncogene 2015; 34: 3640–3650.
Kohlhapp FJ, Mitra AK, Lengyel E, Peter ME . MicroRNAs as mediators and communicators between cancer cells and the tumor microenvironment. Oncogene 2015; 34: 5857–5868.
Ding G, Zhou L, Qian Y, Fu M, Chen J, Chen J et al. Pancreatic cancer-derived exosomes transfer miRNAs to dendritic cells and inhibit RFXAP expression via miR-212-3p. Oncotarget 2015; 6: 29877–29888.
Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T . Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem 2013; 288: 10849–10859.
Yohena T, Yoshino I, Takenaka T, Kameyama T, Ohba T, Kuniyoshi Y et al. Upregulation of hypoxia-inducible factor-1alpha mRNA and its clinical significance in non-small cell lung cancer. J Thorac Oncol 2009; 4: 284–290.
Koshikawa N, Iyozumi A, Gassmann M, Takenaga K . Constitutive upregulation of hypoxia-inducible factor-1alpha mRNA occurring in highly metastatic lung carcinoma cells leads to vascular endothelial growth factor overexpression upon hypoxic exposure. Oncogene 2003; 22: 6717–6724.
Branco-Price C, Zhang N, Schnelle M, Evans C, Katschinski DM, Liao D et al. Endothelial cell HIF-1α and HIF-2α differentially regulate metastatic success. Cancer Cell 2012; 21: 52–65.
Yamakawa M, Liu LX, Date T, Belanger AJ, Vincent KA, Akita GY et al. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res 2003; 93: 664–673.
Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM et al. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J Cell Biol 2015; 208: 821–838.
Li X, Padhan N, Sjöström EO, Roche FP, Testini C, Honkura N et al. VEGFR2 pY949 signalling regulates adherens junction integrity and metastatic spread. Nat Commun 2016; 7: 11017.
Tichet M, Prod’Homme V, Fenouille N, Ambrosetti D, Mallavialle A, Cerezo M et al. Tumour-derived SPARC drives vascular permeability and extravasation through endothelial VCAM1 signalling to promote metastasis. Nat Commun 2015; 6: 6993.
Galaup A, Cazes A, Le Jan S, Philippe J, Connault E, Le Coz E et al. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc Natl Acad Sci USA 2006; 103: 18721–18726.
Rodriguez PL, Jiang S, Fu Y, Avraham S, Avraham HK . The proinflammatory peptide substance P promotes blood-brain barrier breaching by breast cancer cells through changes in microvascular endothelial cell tight junctions. Int J Cancer 2014; 134: 1034–1044.
Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 2009; 136: 839–851.
Acknowledgements
This study was supported by grants from the Ministry of Science and Technology (MOST 104-2314-B-037-053-MY4; MOST 104-2320-B-037-014-MY3; MOST 103-2320-B-037-006-MY3), and the Kaohsiung Medical University ‘Aim for the Top Journals Grant’ (Grant No. KMU-DT106005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Hsu, YL., Hung, JY., Chang, WA. et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene 36, 4929–4942 (2017). https://doi.org/10.1038/onc.2017.105
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.105
This article is cited by
-
Co-culture engineering: a promising strategy for production of engineered extracellular vesicle for osteoarthritis treatment
Cell Communication and Signaling (2024)
-
Tumor-derived small extracellular vesicles in cancer invasion and metastasis: molecular mechanisms, and clinical significance
Molecular Cancer (2024)
-
Extracellular vesicles as tools and targets in therapy for diseases
Signal Transduction and Targeted Therapy (2024)
-
Roles of exosomes in immunotherapy for solid cancers
Cell Death & Disease (2024)
-
Insights into the complex interactions between Rab22a and extracellular vesicles in cancers
Inflammation Research (2024)