Abstract
When cells in G2 phase are challenged with DNA damage, several key mitotic regulators such as Cdk1/Cyclin B, Aurora A and Plk1 are inhibited to prevent entry into mitosis. Here we have studied how inhibition of Plk1 is established after DNA damage. Using a Förster resonance energy transfer (FRET)-based biosensor for Plk1 activity, we show that inhibition of Plk1 after DNA damage occurs with relatively slow kinetics and is entirely dependent on loss of Plk1-T210 phosphorylation. As T210 is phosphorylated by the kinase Aurora A in conjunction with its co-factor Bora, we investigated how they are affected by DNA damage. Interestingly, we find that the interaction between Bora and Plk1 remains intact during the early phases of the DNA damage response (DDR), whereas Plk1 activity is already inhibited at this stage. Expression of an Aurora A mutant that is refractory to inhibition by the DDR failed to prevent inhibition of Plk1 and loss of T210 phosphorylation, suggesting that inhibition of Plk1 may be established by perturbing recruitment of Aurora A by Bora. Indeed, expression of a fusion in which Aurora A was directly coupled to Bora prevented DNA damage-induced inhibition of Plk1 activity, as well as inhibition of T210 phosphorylation. Taken together, these data demonstrate that DNA damage affects the function of Aurora A at multiple levels: both by direct inhibition of Aurora A activity, as well as by perturbing the interaction with its co-activator Bora. We propose that the DDR targets recruitment of Aurora A to the Plk1/Bora complex to prevent activation of Plk1 during DNA damage in G2.
Similar content being viewed by others
Introduction
One of the most life-threatening events that can occur to cells that are preparing to divide is a double-stranded break in their DNA. In order to deal with such an event, cells activate a DNA damage-dependent checkpoint, the DNA damage response (DDR), which results in a cell cycle arrest.1 This arrest provides cells with time to repair the damaged DNA and ensures that cells can enter mitosis with an intact genome, or initiate apoptosis or senescence when the damage is too extensive.2 To prevent cells from entering mitosis, the DDR can repress the pro-mitotic machinery that leads to activation of Cdk1/Cyclin B.3 One of the key targets of the DNA damage checkpoint is Plk1.4, 5 Plk1 is involved in the activation of Cdk1, but also controls repair and is required to restart the cell cycle following a DNA damage-induced arrest, a process called checkpoint recovery.5, 6 Activation of Plk1 starts in G2, ~5–6 h before mitotic entry.7 At this time, Plk1 is phosphorylated on T210 in its T-loop by Aurora A, resulting in activation of the Plk1 kinase domain.7, 8, 9 Phosphorylation of Plk1 at T210 by Aurora A requires binding of the co-factor Bora.7, 9 During G2, Plk1 and Bora form a complex, which is initiated by Cdk1 activity10, 11, 12 and leads to initial Plk1 activation in the nucleus.13
When cells are challenged with genotoxic stress such as double-strand breaks in the G2 phase of the cell cycle, activity of Plk1 is inhibited4 and Plk1 degradation is induced.14 In addition, upstream activators of Plk1 are similarly affected by the DDR; Aurora A activity is inhibited in a Chk1-dependent manner,15 whereas Bora has been shown to be targeted by ATR for degradation in a β-TrCP-dependent manner after ultraviolet-induced DNA damage.16 Moreover, activation of the DDR results in inhibition of Cdk-activity,17 which normally promotes the binding of Bora to Plk1.10, 11 Thus, activation of Plk1 seems to be prevented at multiple levels after DNA damage, possibly to enforce tight inhibition of its activity.
Controlling Plk1 activity during the DDR is especially important, as Plk1 can promote recovery from the DNA damage-induced arrest, not only through re-activation of the cell cycle machinery6 but Plk1 can also silence signaling of the DDR at multiple levels. Plk1 was shown to inhibit localization of 53BP1 to DNA damage foci, to promote the inhibition of Chk2 as well as the degradation of the Chk1 activator Claspin.6, 18, 19, 20, 21 Interestingly, although Plk1 activity is actively repressed during the DDR, its activity seems to be required for efficient repair, as Plk1-mediated phosphorylation was shown to recruit Rad51 to sites of damage, to facilitate homologous recombination.22 These observations suggest that intricate regulation of Plk1 activity is required during the DDR to coordinate repair, checkpoint silencing and cell cycle re-entry.
To gain more insight into the regulation of Plk1, we studied how inhibition of Plk1 is established in response to DNA damage. Here we show that Plk1 activity is first inhibited through dephosphorylation of T210. This initial decrease in T210 phosphorylation on DNA damage is not paralleled by a disruption of the Plk1/Bora complex, the formation of which is an essential step towards activation of Plk1.7, 9 This observation suggests that the inhibition of Plk1 is not a mere reversal of its activation through Cdk-dependent Bora complex formation. In addition, although Aurora A activity is rapidly lost after DNA damage, expression of a constitutively active mutant of Aurora A failed to overcome inhibition of T210 phosphorylation following activation of the DNA damage checkpoint. We were able to show that forced recruitment of Aurora A to Plk1 by directly fusing Aurora A to Bora could circumvent the inhibition of Plk1 on DNA damage. We propose that preventing recruitment of Aurora A to the Plk1/Bora complex, in addition to control of Aurora A activity itself, is required to silence Plk1 activity during the early stages of a DNA damage-induced arrest and to prevent premature activation of the pro-mitotic machinery.
Results
Dephosphorylation of the T210 residue is required for inhibition of Plk1 activity by the DDR
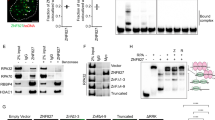
Activation of the DDR leads to loss of phosphorylated T210 and inhibition of Plk1 activity.4 During the G2 phase of the cell cycle, Plk1 gets phosphorylated on T210; thus, we asked whether this phosphorylation is removed when DNA damage is inflicted on cells that already contain T210-phosphorylated Plk1. To this end, cells were released from a thymidine block for 8 h, a time point at which T210 phosphorylation is first detected (Figure 1a and Supplementary Figure S1A). Although 3 h of mock treatment caused a further increase in the amount of phosphorylated T210, induction of DNA damage by etoposide at this time causes a reduction in T210 phosphorylation (Figure 1a, compare lanes 1 and 3). This observation indicates that activation of the DDR blocks T210 phosphorylation and results in dephosphorylation of previously phosphorylated Plk1. The effect of DNA damage on T210 phosphorylation could be overcome by inhibition of ATR or Chk1 but not ATM or Chk2 (Figure 1a).
Plk1 is inhibited after the DNA damage checkpoint through regulation of T210 phosphorylation. (a) U2OS cells were synchronized in G2 by an 8 h thymidine release. After 8 h, cells were treated as indicated. Plk1 was immunprecipitated and samples were probed with the indicated antibodies. (b) Asynchronously growing U2OS cells expressing the Plk1 FRET-based biosensor were filmed and at time point 0 the indicated drugs were added. Cyan fluorescent protein/yellow fluorescent protein (CFP/YFP) emission ratios were calculated and plotted over time. Stills show representative cells in each of the different conditions. Graphs depict analysis of 10 individual cells. Cells that entered mitosis before addition of the drugs are indicated in blue and cells that had active Plk1 at the time of drug addition are shown in red. Dashed gray lines indicate time point zero. (c) The Plk1 FRET-based biosensor was transiently transfected in cells stably expressing tetracycline-inducible Plk1-wt or Plk1-T210D. Expression was induced with tetracycline and individual cells were treated and analyzed as in b.
Next, we looked closer at the kinetics of Plk1 inhibition after DNA damage using a Förster resonance energy transfer (FRET)-based biosensor for Plk1 that allows us to monitor Plk1 activity in real time in living cells.7, 23 We first analyzed asynchronous cycling U2OS cells by time-lapse fluorescence microscopy to monitor Plk1 activity over time. Using the increase of the cyan fluorescent protein/yellow fluorescent protein (CFP/YFP) ratio as a measure for Plk1 activity, we are able to identify cells that are in the G2 phase of the cell cycle and are preparing to enter mitosis. This allowed us to observe what happens to Plk1 activity in cells that are in different stages of G2 (based on the CFP/YFP-ratio) and therefore have already established different levels of Plk1 activation (Figure 1b). Addition of the Plk1 inhibitor BI 253624 resulted in an almost immediate decrease in Plk1 activity, reaching basal levels in approximately half an hour. This is in accordance with our previous observations with BI 2536 in mitotic cells.25 Addition of etoposide to these G2 cells resulted in an immediate block in the further activation of Plk1, followed by a progressive decrease in Plk1 activity that was decidedly slower than inhibition of Plk1 by BI 2536. Interestingly, even the cells that were on the brink of entering mitosis at the moment of etoposide addition (judged by the level of Plk1 activity) were able to arrest in G2 (Figure 1b). The effect of etoposide on Plk1 activity was completely overridden when Chk1 was inhibited by addition of UCN-01, consistent with our observation that etoposide-induced inhibition of T210 phosphorylation requires Chk1. These results show that Plk1 activation is immediately blocked in response to activation of the DNA damage checkpoint, while further inhibition of Plk1 activity is a slower process.
In addition to regulation of T-loop phosphorylation, Plk1 is degraded in response to DNA damage.14 To investigate the possible contribution of Plk1 degradation to its inactivation in response to DNA damage, we added the proteasome inhibitor MG-132 after induction of DNA damage to prevent its degradation. When monitoring Plk1 activity after addition of etoposide in the absence or presence of MG-132, we observed that Plk1 activity was similarly inhibited, regardless of whether Plk1 was stabilized or not (Supplementary Figure S1B). In addition, phosphorylation of Plk1 at T210 after stabilizing Plk1 following DNA damage did not differ from the control samples (Supplementary Figure S1C). These observations suggest that proteasomal degradation does not control Plk1 activity at the onset of the DDR but rather has effects during the later stages of the DDR.
Next, we asked whether regulation of T210 phosphorylation is sufficient to inactivate Plk1 following DNA damage or whether other DNA damage-dependent phosphorylation sites on Plk1 might contribute to inactivation of Plk1, independent of T210 phosphorylation. To test this, we monitored Plk1 activity in cells expressing wild-type Plk1 or a phosphorylation-mimicking T210D mutant of Plk1, previously shown to be fully active throughout G2.7 In cells expressing wild-type Plk1, we could clearly observe the inactivation of Plk1 in response to DNA damage (Figure 1c). However, in cells expressing the Plk1-T210D mutant, addition of etoposide did not affect Plk1 activity, even though an arrest was induced that prevented cells from entering mitosis (Figure 1c). Taken together, these results show that Plk1 activity is strictly controlled via phosphorylation of the T-loop at T210 in response to DNA damage.
Inhibition of Plk1 activity after DNA damage occurs independently of Plk1/Bora complex disassembly
Phosphorylation of Plk1 at T210 during the G2 phase of the cell cycle is carried out by Aurora A.7, 9 In order for Aurora A to phosphorylate Plk1, the co-factor Bora needs to form a complex with Plk1.7, 9 Binding of Bora to Plk1 depends on Cdk-dependent phosphorylation of Bora.10, 11 As Cdk activity is known to be inhibited after DNA damage,17 we reasoned that the Plk1/Bora complex could be disrupted in response to DNA damage. Therefore, we investigated the formation of this complex before and after damaging cells in G2. To test the roles these individual kinases have in formation of the Plk1/Bora complex, we synchronized cells in G2, inflicted DNA damage and subsequently induced recovery by adding caffeine 16 h after the damage. During recovery we treated cells with several small molecule inhibitors that are specific for Plk1 (BI 2536), Aurora A (MLN 8054), Cdk1 (RO 3306) or a more general Cdk inhibitor (Roscovitine).24, 26, 27, 28 Induction of recovery resulted in formation of the Plk1/Bora complex and activation of Plk1 by phosphorylation of T210. Inhibition of Plk1 or Aurora A using BI 2536 or MLN 8054, respectively, resulted in reduced T210 phosphorylation (Figure 2a). However, the complex between Plk1 and Bora was still formed, indicating that Plk1/Bora complex formation occurs independently of Plk1 and Aurora A activity. In accordance with the literature, inhibition of Cdk activity resulted in both loss of T210 phosphorylation and inhibition of Plk1/Bora complex formation (Figure 2a). In fact, we could observe a reduction in the total protein levels of Bora on inhibition of Cdk activity. These observations reiterate that Cdk activity is required for formation of the Plk1/Bora complex during recovery. To test whether Cdk1 inhibition or DNA damage can revert Plk1 activation and Plk1/Bora complex formation once Plk1 is activated, we synchronized cells in G2 and added the Cdk1 inhibitor RO 3306, adriamycin or etoposide, to inhibit phosphorylation of T210 on Plk1. Induction of DNA damage resulted in loss of T210 phosphorylation (Figure 2b). However, inhibition of Cdk1 by RO 3306 did not seem to result in a loss of T210 phosphorylation, despite a reduction in the amount of Bora bound to Plk1.
Plk1 and Bora remain in a complex during the initial stages of the DDR. (a) U2OS cells were synchronized by thymidine, released for 7 h in G2 and treated for 1 h with 0.5 μM adriamycin. Cells remained arrested for 16 h and recovery was induced by addition of caffeine. During caffeine treatment, cells were co-treated with the indicated inhibitors. Plk1 was immunoprecipitated and protein levels were analyzed by western blotting. (b) U2OS cells were synchronized by thymidine and released into G2 for 8 h. Where indicated, cells were treated with 1 h 0.5 μM adriamycin or 10 μM etoposide and collected after a total of 11 h after thymidne release or for 3 h with 4 μM RO 3306. Plk1 was immunoprecipitated and protein levels were analyzed by western blotting. (c) U2OS cells were released after thymidine release (TR) at the indicated times and treated with the indicated types of DNA damage (0.5 μM adriamycin 10 μM etoposide or 10 Gy of ionizing irradiation (IR)). Plk1 was immunprecipitated and samples were probed with the indicated antibodies.
To determine how the Plk1/Bora complex was affected directly after induction of DNA damage, we immunoprecipitated Plk1 and probed for binding to Bora. As expected, phosphorylation of T210 decreased after induction of DNA damage either by adriamycin, etoposide or 10 Gy of ionizing irradiation (Figure 2c). This reduction was already observed at 3 h after damage induction and persisted up to 16 h after the damaging insult in the etoposide- and adriamycin-treated cultures (Figure 2c). In the irradiated cultures, we consistently observed reappearance of T210 phosphorylation, which is likely to be due to the fact that a fraction of cells have recovered at 16 h after ionizing irradiation. At 16 h into the arrest, we could observe a decrease in the levels of both Bora and Plk1, as described before.14, 16 Interestingly, although Plk1/Bora complex formation was clearly reduced 16 h after DNA damage, the complex seemed to be unaffected by the damage at 3 h (Figure 2c). In fact, we could observe a clear increase in the amount of Bora bound to Plk1 when comparing samples taken before (8 h, lane 1 and 2, Figure 2c) and after DNA damage (lanes 3–5, Figure 2c).
Taken together, these data reiterate that Cdk activity is at the basis of Plk1/Bora complex formation and subsequent Plk1 activation. However, once the complex is formed and Plk1 is at least partially activated, inhibition of Cdk1 is insufficient to reverse this process efficiently, suggesting that inhibition of Plk1 during DNA damage does not primarily depend on the loss of Cdk1 activity. This suggests that new complexes of Plk1 and Bora continue to form during the initial phase of the DDR. These results also demonstrate that the inhibition of Plk1 in the first hours following the DNA damage occurs independently of Plk1/Bora complex disassembly or degradation of Bora.
Preventing Aurora A inhibition is insufficient to fully sustain Plk1 activity after DNA damage
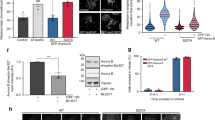
As we find that Plk1 and Bora remain in a complex at a time when T210 phosphorylation is clearly decreased, we wondered whether the initial inhibition of Plk1 activity is entirely due to inhibition of Aurora A during the early stages of the DDR. Indeed, when using Aurora A-T288 autophosphorylation as a readout of Aurora-A activity,29 we found that Aurora A activity was reduced at 3 h after DNA damage in G2 both in endogenous and exogenous versions of Aurora A (Figure 3a and Supplementary Figure S2A), albeit that the relative reduction was much lower than what we observe for Plk-T210 phosphorylation, indicating some residual activity (Figure 3a). Next, we generated stable cell lines expressing FLAG-tagged Aurora A under control of a tetracycline-regulatable promoter. We obtained such cell lines for wild-type Aurora A, a kinase-dead K162R mutant and a constitutively active T288E mutant (Figure 3b), and determined kinase activity of the different variants by in vitro kinase assays. As expected, wild-type Aurora A and the T288E mutant immunoprecipitated from mitotic cells were highly active, whereas the K162R-mutant displayed very little activity (Figure 3c). Importantly, the T288E mutant was fully active in damaged cells, a condition where kinase activity of wild-type Aurora A is similar to the level of the kinase-dead K162R mutant (Figure 3c).
Aurora A activity is not sufficient to activate Plk1 during the DDR. (a) Cells were released from a single thymidine block as indicated and treated for 1 h with 10 μM etoposide at the indicated time. Aurora A and Plk1 were immunoprecipitated and samples were blotted for the indicated proteins. (b) UT2R cells stably expressing tetracycline-inducible FLAG-Aurora A-wt, -K162R or -T288E were treated over night with tetracycline where indicated and expression was anlyzed by western blotting. (c) FLAG-Aurora A-wt, -K162R or -T288E from tetracycline-inducible U2TR cells were immunoprecipitated from a population of mitotic cells (mitotic shake-off) or DNA damage arrested cells (DNA damage) and assayed for activity using recombinant H3 as a substrate and probed for phosphorylation at S10. Lanes were present on the same blot but pasted together for comparison as indicated by the dashed lines. (d) U2TR cells expressing tetracycline-inducible FLAG-Aurora A-T288E were treated as indicated and blotted for the indicated proteins. (e) The Plk1 FRET-based biosensor was transiently transfected in cells stably expressing tetracycline-inducible Plk1-wt, Plk1-T210D, Aurora A-wt or Aurora A-T288E. Expression was induced with tetracycline and individual cells were treated as in 1B.
Next, we induced DNA damage in the Aurora A-T288E-expressing cell line and immunoprecipitated Plk1 (Figure 3d). When Aurora A-T288E expression was induced we could not sustain phosphorylation of T210 after DNA damage, indicating that persistent Aurora A activity in damaged cells is insufficient to prevent dephosphorylation of Plk1 at T210. These results were reiterated when the RNA interference-insensitive Aurora A-T288 mutant was expressed in a background where endogenous Aurora A was depleted (Supplementary Figure S2B). Finally, we transfected the FRET-based biosensor in our Myc-Plk1-wt-, Myc-Plk1-T210D-, FLAG-Aurora A-wt- and FLAG-Aurora A-T288E-inducible cell lines and monitored Plk1 activity in G2 and after DNA damage (Figure 3e). As observed previously, wild-type Plk1 was inhibited after induction of DNA damage, whereas Plk1-T210D remained highly active, even after damage. In line with the western blot analysis, cells expressing Aurora A-wt or Aurora-T288E did not show any significant delay on Plk1 inactivation on induction of the DDR (Figure 3e). Therefore, we can conclude that, although overexpression of a constitutively active Aurora A-T288E mutant clearly contributes to Plk1 activity, it is not sufficient to circumvent Plk1 inhibition during the DDR.
Bora recruits Aurora A to the Plk1/Bora complex to activate Plk1
As we observed that sustained Aurora A activity can not prevent T210 dephosphorylation during the DDR, we reasoned there must be other mechanisms, possibly involving Bora, which contribute to this process. Therefore, we wanted to establish whether Bora is an essential component of this process. Indeed, knockdown of endogenous Bora by small interfering RNAs in our system similarly resulted in a reduction of T210-phosphorylated Plk1 (Figure 4a) in line with what has been reported.7, 9 As expression of constitutively active Aurora A-T288E did not result in increased Plk1 phosphorylation of T210 or kinase activity, we reasoned that during the DDR, Aurora A might not have access to the T210 site rather than having its kinase activity inactivated. Despite our best efforts, we were unable to co-immunoprecipitate Aurora A with the Plk1/Bora complex, suggesting a very transient recruitment of Aurora A to the complex (data not shown). Therefore, we decided to test whether forced complex formation between Aurora A and Bora could overcome the DNA damage-induced inhibition of Plk1. To this end, we constructed two FLAG-tagged fusion proteins, one in which Aurora A was directly fused to the N-terminus of Plk1 and one in which Aurora A was directly fused to the N-terminus of Bora, namely FLAG-AurA-Plk1 and FLAG-AurA-Bora, respectively (Figure 4b). Both constructs were still recognized by their respective antibodies (Figure 4b) and were located predominantly in the cytoplasm, where endogenous Plk1, Aurora A and Bora are also located (Figure 4c).5, 13, 30
Recruitment of Aurora A to the Plk1/Bora complex is the critical step in Plk1 activation. (a) U2OS cells were transfected with control small interfering RNAs (siRNAs) and siRNAs targeting Bora, synchronized by thymidine release in G2 and treated with adriamycin. Samples were collected for total lysates and immunoprecipitated Plk1 and analyzed by western blotting for the indicated antibodies. (b and c) U2OS cells were transfected with the indicated constructs and analyzed by western blot (b) or immunofluorescence (c) with the indicated antibodies. (d and e) U2OS cells expressing the indicated constructs were synchronized in G2 by thymidine release and treated as indicated. Samples were collected for total lysates and immunoprecipitated Plk1 and analyzed by western blotting using the indicated antibodies; s.e., short exposure; l.e., long exposure. (f) The Plk1 FRET-based biosensor was transiently transfected in wt cells or in cells expressing FLAG-AurA-Bora or Plk1-T210D. Stills show representative cells in each of the different conditions. Graphs depict analysis of 10 individual cells. Individual cells were treated and analyzed as in b.
To test the effect of these constructs on the kinetics of Plk1 inhibition during G2 and the DDR, we performed a thymidine block release in cells expressing these constructs and induced damage when the cells were in the G2 phase of the cell cycle (Figure 4d). The FLAG-AurA-Plk1-expressing cells displayed T210 phosphorylation on Plk1, as cells entered G2 and loss of T210 phosphorylation on DNA damage, similar to the empty vector control samples. In addition, phosphorylation of the construct itself on the corresponding T210 site behaved similar to endogenous Plk1, showing a decrease on induction of DNA damage (Supplementary Figure S2C). In contrast, the cells expressing FLAG-AurA-Bora displayed a dramatic increase in total T210 phosphorylation levels during G2 and these levels were unaffected by induction of DNA damage (Figure 4d). These effects are corroborated when we compared FLAG-AurA-Bora with exogenous wild-type FLAG-Aurora A (Figure 4e). We find that our RNA interference-insensitive fusion constructs similarly affect the phosphorylation of T210 in a background where endogenous Aurora A or endogenous Bora are depleted by RNA interference (Supplementary Figure S2D–F).
Strikingly, we observe these effects on phosphorylation of T210, while using the wild-type version of Aurora A in our FLAG-AurA-Bora fusion construct. As phosphorylation of Plk1 at T210 requires an active Aurora A, we asked whether the T288 on Aurora A was phosphorylated in our fusion construct. Indeed we find that, similar to phosphorylation of T210 on Plk1, T288 phosphorylation was dramatically increased compared with FLAG-Aurora A both in an unperturbed G2 and after activation of the DNA damage checkpoint (Supplementary Figure S2G). This observation indicates that once Aurora A is forced to the Plk1/Bora complex, Aurora A becomes highly active, indicating activating feedback loops within the complex.
To monitor the effects of FLAG-AurA-Bora on Plk1 activity, we transfected the construct in cells that co-expressed the Plk1-specific FRET-based biosensor. Whereas empty vector-transfected control cells showed a clear inhibition of Plk1 activity on induction of DNA damage and the Plk1-T210D-expressing cells displayed constitutive Plk1 activity, the cells expressing our FLAG-AurA-Bora fusion construct showed an intermediate effect; the cells had a higher base level of nuclear Plk1 activity and this level of activity was unaffected by invoking the DDR (Figure 4f). Taken together, these data suggest that abrogation of Aurora A recruitment to the Plk1/Bora complex during the early stages of the DDR is an important additional step to inhibit Plk1 activity. The fact that fusion of Aurora A directly to Plk1 does not seem to affect Plk1 T210 phosphorylation suggests that the recruitment of Aurora A is mediated by Bora through an as yet unidentified mechanism.
Discussion
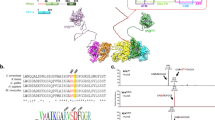
During mitotic entry, Plk1 is activated in G2 by phosphorylation of the T210 residue present in its T-loop. This phosphorylation is executed by Aurora A and requires complex formation of Plk1 with Bora.7, 9 Although activation of Plk1 through this complex has been extensively studied, relatively little is known about complex dissociation on DNA damage. Here we show that on DNA damage, inhibition of Plk1 is mediated entirely through dephosphorylation of the T210 residue. However, the complex of Plk1 and Bora does not fall apart in the early phases of the DDR, nor does constitutive Aurora A activation during a DDR suffice to prevent T210 dephosphorylation. Instead, we show that forced association of Aurora A to the Plk1/Bora complex completely restores T210 phosphorylation on activation of the DDR (summarized in Figure 5). Based on our data, we hypothesize that during the initial stages of the DDR, Plk1 activity is inhibited by perturbing Aurora A recruitment to the Plk1/Bora complex.
The DDR prevents Aurora A recruitment to the Plk1/Bora complex. Just before mitotic entry in G2 Aurora A is recruited to the Plk1/Bora complex and phosphorylates Plk1 at T210 (panel 1). During DNA damage, recruitment of Aurora A to the Plk1/Bora complex is prevented (panel 2). Expression of a constitutively active Aurora A-T288E mutant is unable to phosphorylate Plk1 at T210 (panel 3). Forced association of Aurora A to Bora by fusing both proteins together during the DDR results in phophorylation of Plk1 at T210 and subsequent activation (panel 4).
On induction of the DDR, the interaction between Bora and Plk1 remains intact for several hours but T210 phosphorylation is lost. Thus, loss of T210 phosphorylation is not a consequence of a disruption of the Plk1/Bora interaction. However, direct fusion of Bora and Aurora A can prevent Plk1 inhibition by DNA-damaging agents, indicating that the DDR targets the interaction between Aurora A and Bora to establish inhibition of Plk1. How exactly the DDR can perturb the functional interplay between Aurora A and Bora is not clear. This is not easily investigated, as our extensive efforts to co-immunoprecipate Aurora A with the Plk1/Bora complex produced negative results, indicating that interaction of Aurora A with the Plk1/Bora complex is highly transient.
It has been known for some time that Plk1 activity is inhibited in response to DNA damage.4 However, one of the most striking things we observed was that complete inhibition of activity of Plk1 after DNA damage took around 5 h, whereas inhibition of Plk1 by addition of a pharmacological inhibitor was complete within approximately half an hour, similar to inactivation of fully active Plk1 in mitosis.25 Thus, cells retain Plk1 activity for a substantial period after the DDR is triggered. This observation indicates that during the DNA damage arrest, cells simply need to prevent Plk1 from becoming fully active. However, retention of Plk1 activity shortly after activation of the DDR might be functionally relevant and indicate that Plk1 activity is required during the early phases of the DDR. In fact, Plk1-mediated phosphorylation was shown to be involved in the recruitment of Rad51 to sites of damage and thus cells could require Plk1 activity to start up the repair process.22 In addition, Plk1 feeds back into the DDR to control duration and shut down through 53BP1, Claspin, Chk2 and p53.18, 19, 31 These negative feedback loops between Plk1 and the DNA damage checkpoint could explain why we observe that Plk1 inhibition is dependent on the extent of DNA damage and the relatively slow shutdown of Plk1 activity once the DDR is activated. It will be interesting to see how these pathways relate to each other and control the duration of the DDR and repair efficiency.
In addition, we find that in the early phases of the DDR, the Plk1/Bora complex is still intact, while T210 phosphorylation and Plk1 activity are lost. This raises the question why this complex is allowed to be intact, as it is such an essential component of promoting Plk1 activation by Aurora A.7, 9 It is tempting to speculate that the relatively slow Plk1 inactivation during the DDR is a direct result of the remaining complex formation of Plk1 and Bora, as the formation and activation during G2 is subject to several feedback loops.3 The continued association of Plk1 and Bora during the earlier phases of the DDR would allow Plk1 to be available for phosphorylation at T210, possibly to set it up for rapid re-activation when the damage is rapidly repaired and cells can quickly re-enter the cell cycle.
It is currently not well understood how Bora exactly promotes T210 phosphorylation by Aurora A. It has been hypothesized that Bora binding to Plk1 could mediate exposure of the T-loop, to allow access of Aurora A to the T210 site.9 However, Bora remains in complex with Plk1 at a time that T210 phosphorylation is already lost, suggesting that this is not very likely, or at least not the only mechanism. Instead, it seems more plausible that Bora can act as a recruiting platform for Aurora A. Given that this does not seem to produce a very stable complex, this would result in a high on/off rate for Aurora A that could be important to retain tight control over Plk1 activation. Importantly, we find that constitutively active Aurora A cannot sustain phosphorylation of Plk1 during the DDR, even though Bora still binds to Plk1. This observation, in combination with our findings that forced recruitment of Aurora A does lead to phosphorylation of T210, further supports the notion that the T210 site is accessible during the DDR, and that Aurora A recruitment to Plk1 is the likely step that is abrogated by the DDR. As our FLAG-AurA-Bora fusion construct contains a wild-type version of Aurora A and not the constitutively active T288E mutant, it is clear that Aurora A fused to Bora is hyperactive in the presence of DNA damage. This hyperactivation of Aurora A when it is forced to be a part of the Plk1/Bora complex indicates that Plk1 and Bora are involved in activating Aurora A. As mentioned before, feedback loops are an integral part of the signaling pathways that lead to mitotic entry and feedback from Plk1/Bora to Aurora A has been reported.10, 32 These observations further stress the importance of interfering with Aurora A recruitment to the Plk1/Bora complex during the DDR. The potential of Aurora A to phosphorylate and activate Plk1 in the presence of DNA damage is a clear reason for the DDR to target the recruitment mechanisms of Aurora A to the Plk1/Bora complex and thus prevent the feedback loops that amplify pro-mitotic signaling pathways.
Although the exact molecular mechanisms of our proposed recruitment would need further confirmation, it is tempting to speculate that recruitment of Aurora A to the Plk1/Bora complex is regulated through phosphoregulation of Bora. First, Bora consists of ~16% serines and 7% threonines, making it very susceptible to phosphorylation-dependent regulation.33 Second, several regulatory sites on Bora have been identified as well as a very prominent phospho-shift.7, 10, 12, 25, 34, 35, 36 Therefore, it is conceivable that the phosphorylation of these sites in Bora might have a role in creating a DDR-sensitive and -reversible docking platform for Aurora A. As recruitment of Aurora A to the Plk1/Bora complex is an important step in this process, it would be interesting to investigate which of the previously identified phosphorylation sites on Bora contribute to Aurora A recruitment or recognition of the Plk1/Bora complex, to provide more insights into the mechanism and dynamics of Plk1 activation. In addition, other proteins such as Fry and CEP192 have been implicated in Aurora A-mediated Plk1 activation and could have an additional role in this process.37, 38
Finally, an important aspect in the regulation of a phosphorylation site is its dephosphorylation. As the phosphorylation of the T210 site in Plk1 decreases on DNA damage, phosphatase-mediated dephosphorylation cannot be disregarded. Several reports have implicated the PP1C regulatory subunit MYPT1 (myosin phosphatase-targeting subunit 1) as the phosphatase for T210 on Plk1.39, 40, 41 However, we have been unable to demonstrate a clear role for dephosphorylation of Plk1 at T210 by MYPT1/PP1C after DNA damage (Supplementary Figure S2H), although it has been suggested.41 Therefore, it remains an intriguing question what the role is of phosphatases in the assembly and disassembly of this complex. We cannot rule out the likely possibility that, in addition to direct regulation of T210 phosphorylation by Aurora A, this kinase might regulate a T210-specific phosphatase. This additional layer of regulation would contribute to the dramatic effects on T210 phosphorylation that we observed. However, regulation of Aurora A recruitment to the Plk1/Bora complex would still be a prerequisite in this scenario.
Recruitment of Aurora A to the Plk1/Bora complex is required for continued Plk1 activation in G2.7, 9 Interestingly, our findings show that the initial inhibition occurs at a different level than inhibition of the Cdk-dependent step that promotes the binding of Plk1 to Bora.10, 11 We propose that disruption of the interaction of Aurora A with the Plk1/Bora complex is used by the DDR to promote the initial inhibition of Plk1, whereas disassembly of the Plk1/Bora at later stages of the DDR helps to promote sustained inhibition of Plk1 activation. Further analysis of the detailed mechanisms will be required to produce a complete picture of the complex regulation of Plk1 during the response to DNA damage.
Materials and Methods
Antibodies, small interfering RNAs and reagents
Phosphospecific Plk1-pT210 was obtained from BD Biosciences (San Jose, CA, USA). Anti-Plk1 was previously described.7 Anti-Plk1 (F8), anti-CDK4 (C-22), anti-Hsp90 (H-114), MYPT1 (E-19) and anti-Actin (I-19) were from Santa Cruz (Heidelberg, Germany). Anti-Aurora A and anti-Aurora A-pT288 were from Cell Signaling (Leiden, The Netherlands). H3-pS10 and H2AX-pS139 were from Millipore BV (Amsterdam, The Netherlands). Anti-FLAG (M2) was from Sigma Aldrich (Zwijndrecht, The Netherlands). The Bora antibody was described previously.25 Secondary antibody Alexa-488 was from Thermo Fisher Scientific (Leiden, The Netherlands) and horseradish peroxidase coupled secondary antibodies from Dako (Heverlee, Belgium). Small interfering RNAs targeting Aurora A (5′-CGGUAGGCCUGAUUGGGUU-3′), Bora (5′-GUGAAGAUGAGGAAGAUAAUU-3′) and MYPT1 ON-TARGETplus siRNA SMARTpools were obtained from Dharmacon (Lafayette, CO, USA). The following drugs were used: ATM inhibitor (10 μM, Millipore BV), ATR inhibitor 45 (10 μM, Medicinal Chemistry Shared Recources, Ohio State University), Adriamycin (0.5 μM Sigma Aldrich), BI 2536 (100 nM, Boehringer Ingelheim, Alkmaar, The Netherlands), Caffeine (5 mM, Sigma Aldrich), Chk1 inhibitor (2.5 μM, Millipore BV), Chk2 inhibitor (10 μM, Sigma Aldrich), Etoposide (indicated concentrations, Sigma Aldrich), MG132 (1 μM, Sigma Aldrich), thymidine (2.5 mM, Sigma Aldrich), Nocodazole (250 ng/ml, Sigma Aldrich), tetracyclin (1 μg/ml, Sigma Aldrich), RO 3306 (10 μM, Millipore BV), Roscovitin (25 μM, Sigma Aldrich) and UCN-01 (0.3 μM, Sigma Aldrich).
Cell culture, cloning, transfections and generation of stable cell lines
Human osteosarcoma U2OS cells were grown in Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific) supplemented with 6% fetal calf serum (Lonza, Breda, The Netherlands), 2 mM L-glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin. Cell lines expressing myc tagged Plk1 wild type, Plk1-T210D mutants under the control of tetracycline-inducible promoter and the cell line stably expressing the FRET-based biosensor were described previously.7 Tetracycline-inducible FLAG-AurA-Bora and FLAG-AurA-Plk1 were generated in the following manner. FLAG-AurA was amplified by PCR using the following primers: 5′-ATGGGTACCATGGATTATAAAGATGATGATGATAAAGACCGATCTAAAGAAAAC-3′ and 5′-ATGGCGGCCGCGTATTCTTTGTTTTGGCAA-3′. The FLAG-AurA PCR product was subsequently cloned into pCDNA4-TO using KpnI and NotI restriction enzymes. Bora and Plk1 were amplified by PCR using the following primers: 5′-ATGGCGGCCGCGGGAGGTGGTGGATCAGGTGGAGGTGGATCTGCGGGACGACACG-3′ and 5′-ATGCTCGAGCTATGGACTGCTGCATTGAAAAGG-3′ for Bora and 5′-ATGGCGGCCGCGGGAGGTGGTGGATCAGGTGGAGGTGGATCTGCTGCAGTGACTGCAGGG-3′ and 5′-ATGCTCGAGTTAGGAGGCCTTGAGACGG-3′ for Plk1, integrating a short N-terminal linker sequence. The resulting PCR products were cloned behind AurA in the pCDNA4-TO-FLAG-AurA construct using NotI and XhoI restriction enzymes. U2TR cells stably expressing tetracycline-inducible FLAG-Aurora A-wt, FLAG-Aurora A-K162R and FLAG-Aurora A-T288E were generated by calcium phosphate transfection, selection of stable clones by zeocin (400 mg/ml, Invitrogen, Breda, The Netherlands) treatment followed by clonal selection. Stable clones were grown in media containing Tet system approved fetal bovine serum (Lonza). For induction of expression, cells were treated for indicated times with tetracycline.
Cell synchronization and DNA damage
Cells were synchronization in G2 by thymidine (24 h) treatment followed by the indicated releases into nocodazole. DNA damage was induced by incubating cells for 1 h with doxorubicin or etoposide or by exposure to a 137Cs source.
Immunoprecipitations, western blotting and kinase assays
Cells were extracted in lysis buffer (2 mM EDTA, 1 mM EGTA, 1% Triton X-100, 0.5 mM dithiothreitol, 5 mM NaF, 20 mM β-glycerophosphate, 0.5 mM Na3VO4 and protease inhibitors), normalized for total protein content and incubated for several hours at 4 °C with polyclonal anti-Plk1 antibody immobilized on protein A (BioRad, Veenendaal, The Netherlands). Immunocomplexes were extensively washed and analyzed by immunoblotting. Samples for western blotting were either prepared in lysis buffer or Laemmli sample buffer and analyzed by immunoblotting. Kinase assays were performed for 20 min at 30 °C in Aurora kinase buffer (50 mM Tris pH 7.5, 15 mM MgCl2, 2 mM EGTA, 0.5 mM and Vanadate 1 mM dithiothreitol) supplemented with 100 μM ATP and 5 μg recombinant H3 (Roche Diagnostics, Almere, The Netherlands). Reactions were prepared for western blotting and probed with anti-H3-pS10. Experiments were repeated at least three times to validate results.
Immunofluorescence and FRET analysis
Fixation and antibody staining for immunofluorescence were performed as described.7 Images show maximum intensity projections of deconvolved Z-stacks, acquired on a Deltavision RT imaging system (GE Healthcare Europe, Eindhoven, The Netherlands) using 0.95 numerical aperture × 20 objectives. The FRET-based probe for monitoring Plk1 activity has been described previously.7 The cyan fluorescent protein/yellow fluorescent protein emission ratio after cyan fluorescent protein excitation of U2OS cells stably expressing the FRET-based biosensor was monitored on a Deltavision Elite imaging system (GE Healthcare Europe), using a × 20 0.75 numerical aperture objective. Images were acquired every 10 or 20 min. The images were processed with ImageJ using the Ratio Plus plug-in (http://rsb.info.nih.gov/ij/). Experiments were repeated at least three times to validate results.
References
Ciccia A, Elledge SJ . The DNA damage response: making it safe to play with knives. Mol Cell 2010; 40: 179–204.
Shaltiel IA, Krenning L, Bruinsma W, Medema RH . The same, only different - DNA damage checkpoints and their reversal throughout the cell cycle. J Cell Sci 2015; 128: 607–620.
Lindqvist A, Rodríguez-Bravo V, Medema RH . The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol 2009; 185: 193–202.
Smits VA, Klompmaker R, Arnaud L, Rijksen G, Nigg EA, Medema RH . Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat Cell Biol 2000; 2: 672–676.
Bruinsma W, Raaijmakers JA, Medema RH . Switching Polo-like kinase-1 on and off in time and space. Trends Biochem Sci 2012; 37: 534–542.
van Vugt MATM Brás A, Medema RH . Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol Cell 2004; 15: 799–811.
Macurek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature 2008; 455: 119–123.
Jang Y-J, Ma S, Terada Y, Erikson RL . Phosphorylation of threonine 210 and the role of serine 137 in the regulation of mammalian polo-like kinase. J Biol Chem 2002; 277: 44115–44120.
Seki A, Coppinger JA, Jang C-Y, Yates JR, Fang G . Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science 2008; 320: 1655–1658.
Chan EHY, Santamaria A, Sillje HHW, Nigg EA . Plk1 regulates mitotic Aurora A function through betaTrCP-dependent degradation of hBora. Chromosoma 2008; 117: 457–469.
Tavernier N, Noatynska A, Panbianco C, Martino L, Van Hove L, Schwager F et al. Cdk1 phosphorylates SPAT-1/Bora to trigger PLK-1 activation and drive mitotic entry in C. elegans embryos. J Cell Biol 2015; 208: 661–669.
Thomas Y, Cirillo L, Panbianco C, Martino L, Tavernier N, Schwager F et al. Cdk1 phosphorylates SPAT-1/Bora to promote Plk1 activation in C. elegans and human cells. Cell Rep 2016; 15: 510–518.
Bruinsma W, Aprelia M, Kool J, Macurek L, Lindqvist A, Medema RH . Spatial separation of Plk1 phosphorylation and activity. Front Oncol 2015; 5: 132.
Bassermann F, Frescas D, Guardavaccaro D, Busino L, Peschiaroli A, Pagano M . The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell 2008; 134: 256–267.
Krystyniak A, Garcia-Echeverria C, Prigent C, Ferrari S . Inhibition of Aurora A in response to DNA damage. Oncogene 2006; 25: 338–348.
Qin B, Gao B, Yu J, Yuan J, Lou Z . ATR regulates DNA damage-induced G2/M checkpoint through the Aurora A cofactor Bora. J Biol Chem 2013; 288: 16139–16144.
Reinhardt HC, Yaffe MB . Phospho-Ser/Thr-binding domains: navigating the cell cycle and DNA damage response. Nat Rev Mol Cell Biol 2013; 14: 563–580.
Mamely I, van Vugt MA, Smits VAJ, Semple JI, Lemmens B, Perrakis A et al. Polo-like kinase-1 controls proteasome-dependent degradation of Claspin during checkpoint recovery. Curr Biol 2006; 16: 1950–1955.
van Vugt MATM, Gardino AK, Linding R, Ostheimer GJ, Reinhardt HC, Ong S-E et al. A mitotic phosphorylation feedback network connects Cdk1, Plk1, 53BP1, and Chk2 to inactivate the G(2)/M DNA damage checkpoint. PLoS Biol 2010; 8: e1000287.
Peschiaroli A, Dorrello NV, Guardavaccaro D, Venere M, Halazonetis T, Sherman NE et al. SCFbetaTrCP-mediated degradation of Claspin regulates recovery from the DNA replication checkpoint response. Mol Cell 2006; 23: 319–329.
Vidanes GM, Sweeney FD, Galicia S, Cheung S, Doyle JP, Durocher D et al. CDC5 inhibits the hyperphosphorylation of the checkpoint kinase Rad53, leading to checkpoint adaptation. PLoS Biol 2010; 8: e1000286.
Yata K, Lloyd J, Maslen S, Bleuyard J-Y, Skehel M, Smerdon SJ et al. Plk1 and CK2 Act in concert to regulate Rad51 during DNA double strand break repair. Mol Cell 2012; 45: 371–383.
Fuller BG, Lampson MA, Foley EA, Rosasco-Nitcher S, Le KV, Tobelmann P et al. Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature 2008; 453: 1132–1136.
Lénárt P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M et al. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol 2007; 17: 304–315.
Bruinsma W, Macurek L, Freire R, Lindqvist A, Medema RH . Bora and Aurora-A continue to activate Plk1 in mitosis. J Cell Sci 2014; 127: 801–811.
Manfredi MG, Ecsedy JA, Meetze KA, Balani SK, Burenkova O, Chen W et al. Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc Natl Acad Sci USA 2007; 104: 4106–4111.
Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H et al. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc Natl Acad Sci USA 2006; 103: 10660–10665.
Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem 1997; 243: 527–536.
Walter AO, Seghezzi W, Korver W, Sheung J, Lees E . The mitotic serine/threonine kinase Aurora2/AIK is regulated by phosphorylation and degradation. Oncogene 2000; 19: 4906–4916.
Feine O, Hukasova E, Bruinsma W, Freire R, Fainsod A, Gannon J et al. Phosphorylation-mediated stabilization of Bora in mitosis coordinates Plx1/Plk1 and Cdk1 oscillations. Cell Cycle 2014; 13: 1727–1736.
Yang X, Li H, Zhou Z, Wang W-H, Deng A, Andrisani O et al. Plk1-mediated phosphorylation of Topors regulates p53 stability. J Biol Chem 2009; 284: 18588–18592.
Hutterer A, Berdnik D, Wirtz-Peitz F, Zigman M, Schleiffer A, Knoblich JA . Mitotic activation of the kinase Aurora-A requires its binding partner Bora. Dev Cell 2006; 11: 147–157.
UniProt Consortium. Activities at the Universal Protein Resource (UniProt). Nucleic Acids Res 2014; 42: D191–D198.
Dephoure N, Zhou C, Villén J, Beausoleil SA, Bakalarski CE, Elledge SJ et al. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA 2008; 105: 10762–10767.
Seki A, Coppinger JA, Du H, Jang C-Y, Yates JR, Fang G . Plk1- and beta-TrCP-dependent degradation of Bora controls mitotic progression. J Cell Biol 2008; 181: 65–78.
Lee Y-C, Liao P-C, Liou Y-C, Hsiao M, Huang C-Y, Lu P-J . Glycogen synthase kinase 3 β activity is required for hBora/Aurora A-mediated mitotic entry. Cell Cycle 2013; 12: 953–60.
Ikeda M, Chiba S, Ohashi K, Mizuno K . Furry promotes Aurora A-mediated Polo-like kinase 1 activation. J Biol Chem 2012; 287: 27670–27681.
Lee K, Rhee K . PLK1 phosphorylation of pericentrin initiates centrosome maturation at the onset of mitosis. J Cell Biol 2011; 195: 1093–1101.
Yamashiro S, Yamakita Y, Totsukawa G, Goto H, Kaibuchi K, Ito M et al. Myosin phosphatase-targeting subunit 1 regulates mitosis by antagonizing polo-like kinase 1. Dev Cell 2008; 14: 787–797.
Kachaner D, Filipe J, Laplantine E, Bauch A, Bennett KL, Superti-Furga G et al. Plk1-dependent phosphorylation of optineurin provides a negative feedback mechanism for mitotic progression. Mol Cell 2012; 45: 553–566.
Chiyoda T, Sugiyama N, Shimizu T, Naoe H, Kobayashi Y, Ishizawa J et al. LATS1/WARTS phosphorylates MYPT1 to counteract PLK1 and regulate mammalian mitotic progression. J Cell Biol 2012; 197: 625–641.
Acknowledgements
We thank L. Macůrek (IMG, Prague) for the mCherry-Bora construct and members of the Medema laboratory for discussions. RM is supported by the Netherlands Genomics Initiative of NWO and by the NWO Gravitation program CancerGenomics.nl. WB, MA, JK and YJX are supported by the Netherlands Genomics Initiative of NWO. IGS was supported with a postdoctoral fellowship from the Basque Country Government (Spain).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Bruinsma, W., Aprelia, M., García-Santisteban, I. et al. Inhibition of Polo-like kinase 1 during the DNA damage response is mediated through loss of Aurora A recruitment by Bora. Oncogene 36, 1840–1848 (2017). https://doi.org/10.1038/onc.2016.347
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.347
This article is cited by
-
Targeting lysine-specific demethylase 1 (KDM1A/LSD1) impairs colorectal cancer tumorigenesis by affecting cancer cells stemness, motility, and differentiation
Cell Death Discovery (2023)
-
Dysregulated G2 phase checkpoint recovery pathway reduces DNA repair efficiency and increases chromosomal instability in a wide range of tumours
Oncogenesis (2021)
-
In search for biomarkers and potential drug targets for uterine serous endometrial cancer
Journal of Cancer Research and Clinical Oncology (2021)
-
BORA regulates cell proliferation and migration in bladder cancer
Cancer Cell International (2020)
-
Quantitative phosphoproteomics to unravel the cellular response to chemical stressors with different modes of action
Archives of Toxicology (2020)