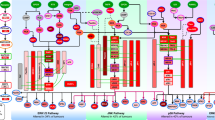
Abstract
During evolution, connections between the major signalling pathways were established to provide cells with an ability to deal with perturbations of homeostasis. However, these feedback and crosstalk mechanisms can become a liability in the treatment of cancer, as the inhibition of one cancer-relevant signalling pathway can lead to the activation of a secondary survival pathway that interferes with cancer drug efficacy. In this review, we discuss connections between signalling pathways in relation to cancer therapy and we evaluate the use of genetic approaches to identify pathway crosstalk. We also discuss how insight into connections between signalling pathways can be exploited to design powerful synthetic lethal drug combination therapies for the treatment of cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008; 455: 1061–1068.
Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012; 487: 330–337.
Alifrangis CC, McDermott U . Reading between the lines; understanding drug response in the post genomic era. Mol Oncol 2014; 8: 1112–1119.
Sun C, Bernards R . Feedback and redundancy in receptor tyrosine kinase signaling: relevance to cancer therapies. Trends Biochem Sci 2014; 39: 465–474.
Groenendijk FH, Bernards R . Drug resistance to targeted therapies: deja vu all over again. Mol Oncol 2014; 8: 1067–1083.
Valachis A, Polyzos NP, Patsopoulos NA, Georgoulias V, Mavroudis D, Mauri D . Bevacizumab in metastatic breast cancer: a meta-analysis of randomized controlled trials. Breast Cancer Res Treat 2010; 122: 1–7.
Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009; 360: 1408–1417.
Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 2009; 360: 563–572.
Ashworth A . A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol 2008; 26: 3785–3790.
Rehman FL, Lord CJ, Ashworth A . Synthetic lethal approaches to breast cancer therapy. Nat Rev Clin Oncol 2010; 7: 718–724.
Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, Nolop K et al. Vemurafenib: the first drug approved for BRAF-mutant cancer. Nat Rev Drug Discov 2012; 11: 873–886.
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364: 2507–2516.
Kopetz S, Desai JD, Chan E, Hecht JR, O'Dwyer PJ, Lee RJ et al. PLX4032 in metastatic colon cancer patients with mutant BRAF tumors. J Clin Oncol 2010; 28. 2010 ASCO Annual Meeting, 15s abstract 3534.
Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012; 483: 100–103.
Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov 2012; 2: 227–235.
Geel RV, Elez E, Bendell JC, Faris JE, Lolkema MPJK, Eskens F et al. Phase I study of the selective BRAFV600 inhibitor encorafenib (LGX818) combined with cetuximab and with or without the α-specific PI3K inhibitor BYL719 in patients with advanced BRAF-mutant colorectal cancer. J Clin Oncol 2014; 32. 2014 ASCO Annual Meeting, 5s suppl; abstr 3514.
Sun C, Wang L, Huang S, Heynen GJ, Prahallad A, Robert C et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature 2014; 508: 118–122.
Girotti MR, Pedersen M, Sanchez-Laorden B, Viros A, Turajlic S, Niculescu-Duvaz D et al. Inhibiting EGF receptor or SRC family kinase signaling overcomes BRAF inhibitor resistance in melanoma. Cancer Discov 2013; 3: 158–167.
Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature 2006; 439: 358–362.
Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol 2008; 26: 2139–2146.
Janne PA, Shaw AT, Pereira JR, Jeannin G, Vansteenkiste J, Barrios C et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol 2013; 14: 38–47.
Migliardi G, Sassi F, Torti D, Galimi F, Zanella ER, Buscarino M et al. Inhibition of MEK and PI3K/mTOR suppresses tumor growth but does not cause tumor regression in patient-derived xenografts of RAS-mutant colorectal carcinomas. Clin Cancer Res 2012; 18: 2515–2525.
Sun C, Hobor S, Bertotti A, Zecchin D, Huang S, Galimi F et al. Intrinsic resistance to MEK inhibition in KRAS mutant lung and colon cancer through transcriptional induction of ERBB3. Cell Rep 2014; 7: 86–93.
Lito P, Saborowski A, Yue J, Solomon M, Joseph E, Gadal S et al. Disruption of CRAF-mediated MEK activation is required for effective MEK inhibition in KRAS mutant tumors. Cancer Cell 2014; 25: 697–710.
Lamba S, Russo M, Sun C, Lazzari L, Cancelliere C, Grernrum W et al. RAF suppression synergizes with MEK inhibition in KRAS mutant cancer cells. Cell Rep 2014; 8: 1475–1483.
Hata AN, Yeo A, Faber AC, Lifshits E, Chen Z, Cheng KA et al. Failure to induce apoptosis via BCL-2 family proteins underlies lack of efficacy of combined MEK and PI3K inhibitors for KRAS-mutant lung cancers. Cancer Res 2014; 74: 3146–3156.
Corcoran RB, Cheng KA, Hata AN, Faber AC, Ebi H, Coffee EM et al. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell 2013; 23: 121–128.
Hatzivassiliou G, Haling JR, Chen H, Song K, Price S, Heald R et al. Mechanism of MEK inhibition determines efficacy in mutant KRAS- versus BRAF-driven cancers. Nature 2013; 501: 232–236.
Dancey JE, Chen HX . Strategies for optimizing combinations of molecularly targeted anticancer agents. Nat Rev Drug Discov 2006; 5: 649–659.
Kummar S, Chen HX, Wright J, Holbeck S, Millin MD, Tomaszewski J et al. Utilizing targeted cancer therapeutic agents in combination: novel approaches and urgent requirements. Nat Rev Drug Discov 2010; 9: 843–856.
Azad NS, Posadas EM, Kwitkowski VE, Steinberg SM, Jain L, Annunziata CM et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol 2008; 26: 3709–3714.
Sosman JA . Improving outcomes in patients with advanced renal cell carcinoma. Expert Rev Anticancer Ther 2008; 8: 481–490.
Lee EQ, Kuhn J, Lamborn KR, Abrey L, DeAngelis LM, Lieberman F et al. Phase I/II study of sorafenib in combination with temsirolimus for recurrent glioblastoma or gliosarcoma: North American Brain Tumor Consortium study 05-02. Neurooncology 2012; 14: 1511–1518.
Garralda E, Paz K, Lopez-Casas PP, Jones S, Katz A, Kann LM et al. Integrated next-generation sequencing and avatar mouse models for personalized cancer treatment. Clin Cancer Res 2014; 20: 2476–2484.
Hidalgo M, Bruckheimer E, Rajeshkumar NV, Garrido-Laguna I, De Oliveira E, Rubio-Viqueira B et al. A pilot clinical study of treatment guided by personalized tumorgrafts in patients with advanced cancer. Mol Cancer Ther 2011; 10: 1311–1316.
Julien S, Merino-Trigo A, Lacroix L, Pocard M, Goere D, Mariani P et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin Cancer Res 2012; 18: 5314–5328.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390.
Rudalska R, Dauch D, Longerich T, McJunkin K, Wuestefeld T, Kang TW et al. In vivo RNAi screening identifies a mechanism of sorafenib resistance in liver cancer. Nat Med 2014; 20: 1138–1146.
Saltz LB, Meropol NJ, Loehrer PJ Sr., Needle MN, Kopit J, Mayer RJ . Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 2004; 22: 1201–1208.
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004; 351: 337–345.
Bardelli A, Siena S . Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol 2010; 28: 1254–1261.
Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012; 486: 532–536.
Albini A, Sporn MB . The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer 2007; 7: 139–147.
De Palma M, Lewis CE . Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013; 23: 277–286.
Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature 2014; 515: 130–133.
Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 2012; 487: 505–509.
Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 2012; 487: 500–504.
Kelly TK, De Carvalho DD, Jones PA . Epigenetic modifications as therapeutic targets. Nat Biotechnol 2010; 28: 1069–1078.
Geutjes EJ, Bajpe PK, Bernards R . Targeting the epigenome for treatment of cancer. Oncogene 2012; 31: 3827–3844.
Thurn KT, Thomas S, Moore A, Munster PN . Rational therapeutic combinations with histone deacetylase inhibitors for the treatment of cancer. Future Oncol 2011; 7: 263–283.
Nebbioso A, Carafa V, Benedetti R, Altucci L . Trials with 'epigenetic' drugs: an update. Mol Oncol 2012; 6: 657–682.
Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB . Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet 1999; 21: 103–107.
Marks PA, Breslow R . Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol 2007; 25: 84–90.
Bertino EM, Otterson GA . Romidepsin: a novel histone deacetylase inhibitor for cancer. Expert Opin Investig Drugs 2011; 20: 1151–1158.
Cai Y, Geutjes EJ, de Lint K, Roepman P, Bruurs L, Yu LR et al. The NuRD complex cooperates with DNMTs to maintain silencing of key colorectal tumor suppressor genes. Oncogene 2014; 33: 2157–2168.
Helming KC, Wang X, Wilson BG, Vazquez F, Haswell JR, Manchester HE et al. ARID1B is a specific vulnerability in ARID1A-mutant cancers. Nat Med 2014; 20: 251–254.
Hoffman GR, Rahal R, Buxton F, Xiang K, McAllister G, Frias E et al. Functional epigenetics approach identifies BRM/SMARCA2 as a critical synthetic lethal target in BRG1-deficient cancers. Proc Natl Acad Sci USA 2014; 111: 3128–3133.
Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 2010; 141: 69–80.
Das Thakur M, Salangsang F, Landman AS, Sellers WR, Pryer NK, Levesque MP et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 2013; 494: 251–255.
Kuczynski EA, Sargent DJ, Grothey A, Kerbel RS . Drug rechallenge and treatment beyond progression—implications for drug resistance. Nat Rev Clin Oncol 2013; 10: 571–587.
Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med 2012; 366: 207–215.
Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N . RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010; 464: 427–430.
Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 2010; 464: 431–435.
Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 2010; 140: 209–221.
Johnson DB, Flaherty KT, Weber JS, Infante JR, Kim KB, Kefford RF et al. Combined BRAF (Dabrafenib) and MEK inhibition (Trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor. J Clin Oncol 2014; 32: 3697–3704.
Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014; 371: 1877–1888.
Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012; 367: 1694–1703.
Packer LM, Rana S, Hayward R, O'Hare T, Eide CA, Rebocho A et al. Nilotinib and MEK inhibitors induce synthetic lethality through paradoxical activation of RAF in drug-resistant chronic myeloid leukemia. Cancer Cell 2011; 20: 715–727.
Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J . Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med 2013; 368: 1365–1366.
Scholl C, Frohling S, Dunn IF, Schinzel AC, Barbie DA, Kim SY et al. Synthetic lethal interaction between oncogenic KRAS dependency and STK33 suppression in human cancer cells. Cell 2009; 137: 821–834.
Luo J, Emanuele MJ, Li D, Creighton CJ, Schlabach MR, Westbrook TF et al. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell 2009; 137: 835–848.
Singh A, Sweeney MF, Yu M, Burger A, Greninger P, Benes C et al. TAK1 inhibition promotes apoptosis in KRAS-dependent colon cancers. Cell 2012; 148: 639–650.
Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature 2009; 462: 108–112.
Acknowledgements
This work was funded by the EU FP7 grant ‘COLTHERES’ and by the Cancer Genomics Netherlands consortium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Prahallad, A., Bernards, R. Opportunities and challenges provided by crosstalk between signalling pathways in cancer. Oncogene 35, 1073–1079 (2016). https://doi.org/10.1038/onc.2015.151
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.151
This article is cited by
-
RNA-binding protein IMP3 is a novel regulator of MEK1/ERK signaling pathway in the progression of colorectal Cancer through the stabilization of MEKK1 mRNA
Journal of Experimental & Clinical Cancer Research (2021)
-
A new precision medicine initiative at the dawn of exascale computing
Signal Transduction and Targeted Therapy (2021)
-
Exploiting synthetic lethality to improve cancer therapy
Nature Reviews Clinical Oncology (2017)