Abstract
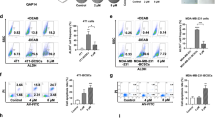
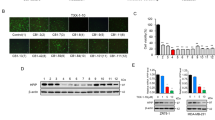
Patients with advanced breast cancer often fail to respond to treatment, creating a need to develop novel biomarkers and effective therapeutics. Dopamine (DA) is a catecholamine that binds to five G protein-coupled receptors. We discovered expression of DA type-1 receptors (D1Rs) in breast cancer, thereby identifying these receptors as novel therapeutic targets in this disease. Strong to moderate immunoreactive D1R expression was found in 30% of 751 primary breast carcinomas, and was associated with larger tumors, higher tumor grades, node metastasis and shorter patient survival. DA and D1R agonists, signaling through the cGMP/protein kinase G (PKG) pathway, suppressed cell viability, inhibited invasion and induced apoptosis in multiple breast cancer cell lines. Fenoldopam, a peripheral D1R agonist that does not penetrate the brain, dramatically suppressed tumor growth in two mouse models with D1R-expressing xenografts by increasing both necrosis and apoptosis. D1R-expressing primary tumors and metastases in mice were detected by fluorescence imaging. In conclusion, D1R overexpression is associated with advanced breast cancer and poor prognosis. Activation of the D1R/cGMP/PKG pathway induces apoptosis in vitro and causes tumor shrinkage in vivo. Fenoldopam, which is FDA (Food and Drug Administration) approved to treat renal hypertension, could be repurposed as a novel therapeutic agent for patients with D1R-expressing tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Amenta F, Ricci A, Tayebati SK, Zaccheo D . The peripheral dopaminergic system: morphological analysis, functional and clinical applications. Ital J Anat Embryol 2002; 107: 145–167.
Rubi B, Maechler P . Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let's seek the balance. Endocrinology 2010; 151: 5570–5581.
Contreras F, Fouillioux C, Bolívar A, Simonovis N, Hernández-Hernández R, Armas-Hernandez MJ et al. Dopamine, hypertension and obesity. J Hum Hypertens 2002; 16: S13–S17.
Li ZS, Schmauss C, Cuenca A, Ratcliffe E, Gershon MD . Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J Neurosci 2006; 26: 2798–2807.
Borcherding DC, Hugo ER, Idelman G, De Silva A, Richtand NW, Loftus J et al. Dopamine receptors in human adipocytes: expression and functions. PLoS One 2011; 6: e25537.
Missale C, Nash SR, Robinson SW, Jaber M, Caron MG . Dopamine receptors: from structure to function. Physiol Rev 1998; 78: 189–225.
Sidhu A, Niznik HB . Coupling of dopamine receptor subtypes to multiple and diverse G proteins. Int J Dev Neurosci 2000; 18: 669–677.
Beaulieu JM, Gainetdinov RR . The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 2011; 63: 182–217.
Arcangeli S, Tozzi A, Tantucci M, Spaccatini C, de Iure A, Costa C et al. Ischemic-LTP in striatal spiny neurons of both direct and indirect pathway requires the activation of D1-like receptors and NO/soluble guanylate cyclase/cGMP transmission. J Cereb Blood Flow Metab 2013; 33: 278–286.
Lin DT, Fretier P, Jiang C, Vincent SR . Nitric oxide signaling via cGMP-stimulated phosphodiesterase in striatal neurons. Synapse 2010; 64: 460–466.
Natarajan A, Han G, Chen SY, Yu P, White R, Jose P . The d5 dopamine receptor mediates large-conductance, calcium- and voltage-activated potassium channel activation in human coronary artery smooth muscle cells. J Pharmacol Exp Ther 2010; 332: 640–649.
Sharma RK, Duda T . Membrane guanylate cyclase, a multimodal transduction machine: history, present, and future directions. Front Mol Neurosci 2014; 7: 56.
Evgenov OV, Pacher P, Schmidt PM, Haskó G, Schmidt HH, Stasch JP . NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov 2006; 5: 755–768.
Azevedo MF, Faucz FR, Bimpaki E, Horvath A, Levy I, de Alexandre RB et al. Clinical and molecular genetics of the phosphodiesterases (PDEs). Endocr Rev 2014; 35: 195–233.
Supuran CT, Mastrolorenzo A, Barbaro G, Scozzafava A . Phosphodiesterase 5 inhibitors—drug design and differentiation based on selectivity, pharmacokinetic and efficacy profiles. Curr Pharm Des 2006; 12: 3459–3465.
Zhu B, Strada SJ . The novel functions of cGMP-specific phosphodiesterase 5 and its inhibitors in carcinoma cells and pulmonary/cardiovascular vessels. Curr Top Med Chem 2007; 7: 437–454.
Zhang L, Zhang Z, Zhang RL, Cui Y, LaPointe MC, Silver B et al. Tadalafil, a long-acting type 5 phosphodiesterase isoenzyme inhibitor, improves neurological functional recovery in a rat model of embolic stroke. Brain Res 2006; 1118: 192–198.
Flaim KE, Gessner GW, Crooke ST, Sarau HM, Weinstock J . Binding of a novel dopaminergic agonist radioligand [3H]-fenoldopam (SKF 82526) to D-1 receptors in rat striatum. Life Sci 1985; 36: 1427–1436.
Murphy MB, Murray C, Shorten GD . Fenoldopam: a selective peripheral dopamine-receptor agonist for the treatment of severe hypertension. N Engl J Med 2001; 345: 1548–1557.
Ng SS, Pang CC . In vivo venodilator action of fenoldopam, a dopamine D(1)-receptor agonist. Br J Pharmacol 2000; 129: 853–858.
Weber RR, McCoy CE, Ziemniak JA, Frederickson ED, Goldberg LI, Murphy MB . Pharmacokinetic and pharmacodynamic properties of intravenous fenoldopam, a dopamine1-receptor agonist, in hypertensive patients. Br J Clin Pharmacol 1988; 25: 17–21.
Bodei S, Arrighi N, Spano P, Sigala S . Should we be cautious on the use of commercially available antibodies to dopamine receptors? Naunyn Schmiedebergs Arch Pharmacol 2009; 379: 413–415.
Michel MC, Wieland T, Tsujimoto G . How reliable are G-protein-coupled receptor antibodies? Naunyn Schmiedebergs Arch Pharmacol 2009; 379: 385–388.
Granados-Principal S, Liu Y, Guevara ML, Blanco E, Choi DS, Qian W et al. Inhibition of iNOS as a novel effective targeted therapy against triple negative breast cancer. Breast Cancer Res 2015; 17: 527.
Shen LH, Liao MH, Tseng YC . Recent advances in imaging of dopaminergic neurons for evaluation of neuropsychiatric disorders. J Biomed Biotechnol 2012 2012. 259349.
Almubarak M, Osman S, Marano G, Abraham J . Role of positron-emission tomography scan in the diagnosis and management of breast cancer. Oncology (Williston Park) 2009; 23: 255–261.
Conole D, Scott LJ . Riociguat: first global approval. Drugs 2013; 73: 1967–1975.
Carlo RD, Muccioli G, Bellussi G, Portaleoni P, Ghi P, Racca S . Steroid, prolactin, and dopamine receptors in normal and pathologic breast tissue. Ann NY Acad Sci 1986; 464: 559–562.
Sachlos E, Risueño RM, Laronde S, Shapovalova Z, Lee JH, Russell J et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell 2012; 149: 1284–1297.
Wang PS, Walker AM, Tsuang MT, Orav EJ, Glynn RJ, Levin R et al. Dopamine antagonists and the development of breast cancer. Arch Gen Psychiatry 2002; 59: 1147–1154.
Zhao DL, Zou LB, Lin S, Shi JG, Zhu HB . Anti-apoptotic effect of esculin on dopamine-induced cytotoxicity in the human neuroblastoma SH-SY5Y cell line. Neuropharmacology 2007; 53: 724–732.
Moreno-Smith M, Lu C, Shahzad MM, Pena GN, Allen JK, Stone RL et al. Dopamine blocks stress-mediated ovarian carcinoma growth. Clin Cancer Res 2011; 17: 3649–3659.
Sarkar C, Chakroborty D, Chowdhury UR, Dasgupta PS, Basu S . Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin Cancer Res 2008; 14: 2502–2510.
Johnson DE, Ochieng J, Evans SL . The growth inhibitory properties of a dopamine agonist (SKF 38393) on MCF-7 cells. Anticancer Drugs 1995; 6: 471–474.
Maggio R, Aloisi G, Silvano E, Rossi M, Millan MJ . Heterodimerization of dopamine receptors: new insights into functional and therapeutic significance. Parkinsonism Relat Disord 2009; 15: S2–S7.
Goldstein DS, Swoboda KJ, Miles JM, Coppack SW, Aneman A, Holmes C et al. Sources and physiological significance of plasma dopamine sulfate. J Clin Endocrinol Metab 1999; 84: 2523–2531.
Eisenhofer G, Coughtrie MW, Goldstein DS . Dopamine sulphate: an enigma resolved. Clin Exp Pharmacol Physiol Suppl 1999; 26: S41–S53.
Ghosh D . Human sulfatases: a structural perspective to catalysis. Cell Mol Life Sci 2007; 64: 2013–2022.
Dajani R, Cleasby A, Neu M, Wonacott AJ, Jhoti H, Hood AM et al. X-ray crystal structure of human dopamine sulfotransferase, SULT1A3. Molecular modeling and quantitative structure-activity relationship analysis demonstrate a molecular basis for sulfotransferase substrate specificity. J Biol Chem 1999; 274: 37862–37868.
Zaccolo M, Movsesian MA . cAMP and cGMP signaling cross-talk: role of phosphodiesterases and implications for cardiac pathophysiology. Circ Res 2007; 100: 1569–1578.
Hoque KE, Indorkar RP, Sammut S, West AR . Impact of dopamine-glutamate interactions on striatal neuronal nitric oxide synthase activity. Psychopharmacology (Berl) 2010; 207: 571–581.
Sammut S, Dec A, Mitchell D, Linardakis J, Ortiguela M, West AR . Phasic dopaminergic transmission increases NO efflux in the rat dorsal striatum via a neuronal NOS and a dopamine D(1/5) receptor-dependent mechanism. Neuropsychopharmacology 2006; 31: 493–505.
Choi BM, Pae HO, Jang SI, Kim YM, Chung HT . Nitric oxide as a pro-apoptotic as well as anti-apoptotic modulator. J Biochem Mol Biol 2002; 35: 116–126.
Proskuryakov SY, Gabai VL . Mechanisms of tumor cell necrosis. Curr Pharm Des 2010; 16: 56–68.
Basu S, Sarkar C, Chakroborty D, Nagy J, Mitra RB, Dasgupta PS et al. Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis. Cancer Res 2004; 64: 5551–5555.
Basu S, Nagy JA, Pal S, Vasile E, Eckelhoefer IA, Bliss VS et al. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med 2001; 7: 569–574.
Chakroborty D, Sarkar C, Basu B, Dasgupta PS, Basu S . Catecholamines regulate tumor angiogenesis. Cancer Res 2009; 69: 3727–3730.
Sarkar C, Chakroborty D, Mitra RB, Banerjee S, Dasgupta PS, Basu S . Dopamine in vivo inhibits VEGF-induced phosphorylation of VEGFR-2, MAPK, and focal adhesion kinase in endothelial cells. Am J Physiol Heart Circ Physiol 2004; 287: H1554–H1560.
Acknowledgements
We dedicate this manuscript to Wilson Tong, M.D., whose untimely death was a major loss to all who knew him. We thank Dean Quaranta for technical assistance, and Drs Susan Waltz and Peter Stambrook for critical reviews of this manuscript. This investigation was funded by NIH grants CA096613 and ES020909, DOD grants AR110050 and BC122992, and pilot grants from Marlene Harris-Ride Cincinnati, and the University of Cincinnati Center for Clinical and Translational Science and Training (CCTST).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Borcherding, D., Tong, W., Hugo, E. et al. Expression and therapeutic targeting of dopamine receptor-1 (D1R) in breast cancer. Oncogene 35, 3103–3113 (2016). https://doi.org/10.1038/onc.2015.369
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.369
This article is cited by
-
Effects of Dopamine on stem cells and its potential roles in the treatment of inflammatory disorders: a narrative review
Stem Cell Research & Therapy (2023)
-
Breast-to-Brain Metastasis: from Microenvironment to Plasticity
Current Breast Cancer Reports (2023)
-
Dopamine receptor D3 is related to prognosis in human hepatocellular carcinoma and inhibits tumor growth
BMC Cancer (2022)
-
QAP14 suppresses breast cancer stemness and metastasis via activation of dopamine D1 receptor
Acta Pharmacologica Sinica (2022)
-
Effect of antipsychotics on breast tumors by analysis of the Japanese Adverse Drug Event Report database and cell-based experiments
Journal of Pharmaceutical Health Care and Sciences (2021)