Abstract
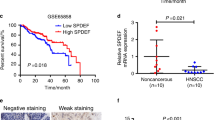
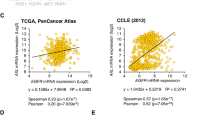
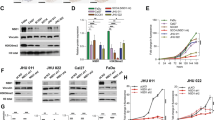
Head and neck squamous cell carcinoma (HNSCC) is an extremely aggressive cancer with a poor prognosis and low patient survival. Because chemotherapy for advanced HNSCC is often ineffective, discovering new therapeutic targets that are important for HNSCC development and progression and elucidating their molecular mechanisms are required. In the present study, we describe the role of DRAK1 (death-associated protein kinase-related apoptosis-inducing kinase 1) as a novel negative regulator of the transforming growth factor-β (TGF-β) tumor suppressor signaling pathway for the first time in human HNSCC cells. DRAK1 was significantly overexpressed in primary human HNSCCs and in HNSCC cell lines. Through gain- and loss-of-function experiments, we demonstrated that the DRAK1 expression level regulated TGF-β1-induced transcriptional activity and expression of the tumor suppressor gene p21Waf1/Cip1. DRAK1 depletion enhanced TGF-β1-induced growth inhibition in vitro and suppressed tumorigenicity in xenograft models in vivo. Mechanistically, DRAK1 was predominantly localized in the cytoplasm and bound to Smad3, thereby interrupting Smad3/Smad4 complex formation, which is the core process for the induction of tumor suppressor genes by TGF-β1. Thus, our findings suggest that cytoplasmic DRAK1 increases tumorigenic potential through inhibition of TGF-β1-mediated tumor suppressor activity in HNSCC cells and may be a potential therapeutic target for HNSCCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Forastiere A, Koch W, Trotti A, Sidransky D . Head and neck cancer. N Engl J Med 2001; 345: 1890–1900.
Hunter KD, Parkinson EK, Harrison PR . Profiling early head and neck cancer. Nat Rev Cancer 2005; 5: 127–135.
Weber F, Xu Y, Zhang L, Patocs A, Shen L, Platzer P et al. Microenvironmental genomic alterations and clinicopathological behavior in head and neck squamous cell carcinoma. JAMA 2007; 297: 187–195.
Koukourakis MI, Bentzen SM, Giatromanolaki A, Wilson GD, Daley FM, Saunders MI et al. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol 2006; 24: 727–735.
Pignon J-P, Al Maître, Maillard E, Bourhis J . Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009; 92: 4–14.
Massague J . TGF-β in cancer. Cell 2008; 134: 215–230.
Massague J, Blain SW, Lo RS . TGF-β signaling in growth control, cancer, and heritable disorders. Cell 2000; 103: 295–310.
Markowitz SD, Roberts AB . Tumor suppressor activity of the TGF-β pathway in human cancers. Cytokine Growth Factor Rev 1996; 7: 93–102.
Kim S-J, Im Y-H, Markowitz S, Bang Y-J . Molecular mechanisms of inactivation of TGF-β receptors during carcinogenesis. Cytokine Growth Factor Rev 2000; 11: 159–168.
Massague J, Seoane J, Wotton D . Smad transcription factors. Genes Dev 2005; 19: 2783–2810.
Levy L, Hill CS . Alterations in components of the TGF-β superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev 2006; 17: 41–58.
Wang D, Song H, Evans JA, Lang JC, Schuller DE, Weghorst CM . Mutation and downregulation of the transforming growth factor beta type II receptor gene in primary squamous cell carcinomas of the head and neck. Carcinogenesis 1997; 18: 2285–2290.
Qiu W, Schönleben F, Li X, Su GH . Disruption of transforming growth factor β-Smad signaling pathway in head and neck squamous cell carcinoma as evidenced by mutations of SMAD2 and SMAD4. Cancer Lett 2007; 245: 163–170.
Bornstein S, White R, Malkoski S, Oka M, Han G, Cleaver T et al. Smad4 loss in mice causes spontaneous head and neck cancer with increased genomic instability and inflammation. J Clin Invest 2009; 119: 3408–3419.
Itoh S, ten Dijke P . Negative regulation of TGF-β receptor/Smad signal transduction. Curr Opin Cell Biol 2007; 19: 176–184.
Watanabe Y, Itoh S, Goto T, Ohnishi E, Inamitsu M, Itoh F et al. TMEPAI, a transmembrane TGF-β-inducible protein, sequesters Smad proteins from active participation in TGF-β signaling. Mol Cell 2010; 37: 123–134.
Yan X, Liu Z, Chen Y . Regulation of TGF-β signaling by Smad7. Acta Biochim Biophys Sin 2009; 41: 263–272.
Kang J, Park S, Kim S, Hong H, Jeong J, Kim H et al. CBL enhances breast tumor formation by inhibiting tumor suppressive activity of TGF-β signaling. Oncogene 2012; 31: 5123–5131.
Sanjo H, Kawai T, Akira S . DRAKs, novel serine/threonine kinases related to death-associated protein kinase that trigger apoptosis. J Biol Chem 1998; 273: 29066–29071.
Bialik S, Kimchi A . The death-associated protein kinases: structure, function, and beyond. Annu Rev Biochem 2006; 75: 189–210.
Mao P, Hever MP, Niemaszyk LM, Haghkerdar JM, Yanco EG, Desai D et al. Serine/theronine kinase 17A is a novel p53 target gene and modulator of cisplatin toxicity and reactive oxygen species in testicular cancer cells. J Biol Chem 2011; 286: 19381–19391.
Mao P, Hever-Jardine MP, Rahme GJ, Yang E, Tam J, Kodali A et al. Serine/threonine kinase 17A Is a novel candidate for therapeutic targeting in glioblastoma. PLoS ONE 2013; 8: e81803.
Yang K-M, Kim W, Bae E, Gim J, Weist BM, Jung Y et al. DRAK2 participates in a negative feedback loop to control TGF-β/Smads signaling by binding to type I TGF-β receptor. Cell Rep 2012; 2: 1286–1299.
Ye H, Yu T, Temam S, Ziober BL, Wang J, Schwartz JL et al. Transcriptomic dissection of tongue squamous cell carcinoma. BMC Genomics 2008; 9: 69.
Pyeon D, Newton MA, Lambert PF, Den Boon JA, Sengupta S, Marsit CJ et al. Fundamental differences in cell cycle deregulation in human papillomavirus–positive and human papillomavirus–negative head/neck and cervical cancers. Cancer Res 2007; 67: 4605–4619.
Peng C-H, Liao C-T, Peng S-C, Chen Y-J, Cheng A-J, Juang J-L et al. A novel molecular signature identified by systems genetics approach predicts prognosis in oral squamous cell carcinoma. PLoS ONE 2011; 6: e23452.
Worsham MJ, Pals G, Schouten J, Van Spaendonk RM, Concus A, Carey TE et al. Delineating genetic pathways of disease progression in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 2003; 129: 129702–129708.
White R, Malkoski S, Wang X . TGF-β signaling in head and neck squamous cell carcinoma. Oncogene 2010; 29: 5437–5446.
Inbal B, Cohen O, Polak-Charcon S, Kopolovic J, Vadai E, Eisenbach L et al. DAP kinase links the control of apoptosis to metastasis. Nature 1997; 390: 180–184.
Kögel D, Prehn JH, Scheidtmann KH . The DAP kinase family of pro‐apoptotic proteins: novel players in the apoptotic game. Bioessays 2001; 23: 352–358.
Jang C-W, Chen C-H, Chen C-C, Chen J-y Su Y-H, Chen R-H . TGF-β induces apoptosis through Smad-mediated expression of DAP-kinase. Nat cell biol 2001; 4: 51–58.
Worsham MJ, Chen KM, Tiwari N, Pals G, Schouten JP, Sethi S et al. Fine-mapping loss of gene architecture at the CDKN2B (p15INK4b), CDKN2A (p14ARF, p16INK4a), and MTAP genes in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 2006; 132: 409–415.
Yang K-M, Jung Y, Lee J-M, Kim W, Cho JK, Jeong J et al. Loss of TBK1 induces epithelial–mesenchymal transition in the breast cancer cells by ERα downregulation. Cancer Res 2013; 73: 6679–6689.
Acknowledgements
This research was supported by the Bio-Synergy Research Project (NRF-2012M3A9C4048735 to S-JK), Bio & Medical Technology Development Program (NRF-2009-0081756 to S-JK) and Basic Science Research Program (NRF-2014R1A1A1005186 to K-MY), through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Park, Y., Kim, W., Lee, JM. et al. Cytoplasmic DRAK1 overexpressed in head and neck cancers inhibits TGF-β1 tumor suppressor activity by binding to Smad3 to interrupt its complex formation with Smad4. Oncogene 34, 5037–5045 (2015). https://doi.org/10.1038/onc.2014.423
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.423