Abstract
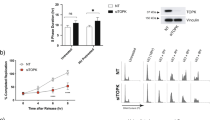
Cdc kinase subunit (Cks) proteins Cks1 and Cks2 are adaptor-like proteins that bind many cyclin-dependent kinases. A wealth of clinical data has shown that Cks proteins are overexpressed in many types of human cancers and this often correlates with increased tumor aggressiveness. Previously, we showed that Cks overexpression abrogates the intra-S-phase checkpoint, a major barrier to oncogene-mediated transformation. Interestingly, the intra-S-phase checkpoint is crucial for the cellular response to replication stress, a major pathway of apoptosis induction by many chemotherapeutic agents. Here, we demonstrate cancer cells that overexpress Cks1 or Cks2 override the intra-S-phase checkpoint in the presence of replication stress-inducing chemotherapies such as 5-Fluorouracil (5-FU) and methotrexate (MTX) leading to enhanced sensitivity in vitro and in vivo. Furthermore, enforced expression of Cks1 in an MTX-resistant breast cancer cell line was found to restore drug sensitivity. Our results suggest that Cks proteins are important determinants of apoptosis induction of replication stress-inducing chemotherapies such as 5-FU.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Reynard GJ, Reynolds W, Verma R, Deshaies RJ . Cks1 is required for G(1) cyclin-cyclin-dependent kinase activity in budding yeast. Mol Cell Biol 2000; 20: 5858–5864.
Patra D, Dunphy WG . Xe-p9, a Xenopus Suc1/Cks homolog, has multiple essential roles in cell cycle control. Genes Dev 1996; 10: 1503–1515.
Patra D, Dunphy WG . Xe-p9, a Xenopus Suc1/Cks protein, is essential for the Cdc2-dependent phosphorylation of the anaphase- promoting complex at mitosis. Genes Dev 1998; 12: 2549–2559.
Murray AW . Recycling the cell cycle: cyclins revisited. Cell 2004; 116: 221–234.
Patra D, Wang SX, Kumagai A, Dunphy WG . The xenopus Suc1/Cks protein promotes the phosphorylation of G(2)/M regulators. J Biol Chem 1999; 274: 36839–36842.
Kaiser P, Moncollin V, Clarke DJ, Watson MH, Bertolaet BL, Reed SI et al. Cyclin-dependent kinase and Cks/Suc1 interact with the proteasome in yeast to control proteolysis of M-phase targets. Genes Dev 1999; 13: 1190–1202.
Harper JW . Protein destruction: adapting roles for Cks proteins. Curr Biol 2001; 11: R431–R435.
Chaves S, Baskerville C, Yu V, Reed SI . Cks1, Cdk1, and the 19S proteasome collaborate to regulate gene induction-dependent nucleosome eviction in yeast. Mol Cell Biol 2010; 30: 5284–5294.
Yu VP, Baskerville C, Grunenfelder B, Reed SI . A kinase-independent function of Cks1 and Cdk1 in regulation of transcription. Mol Cell 2005; 17: 145–151.
Spruck CH, de Miguel MP, Smith AP, Ryan A, Stein P, Schultz RM et al. Requirement of Cks2 for the first metaphase/anaphase transition of mammalian meiosis. Science 2003; 300: 647–650.
Spruck C, Strohmaier H, Watson M, Smith AP, Ryan A, Krek TW et al. A CDK-independent function of mammalian Cks1: targeting of SCF(Skp2) to the CDK inhibitor p27Kip1. Mol Cell 2001; 7: 639–650.
Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A . Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem 2001; 278: 25752–25757.
Tedesco D, Lukas J, Reed SI . The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCF(Skp2). Genes Dev 2002; 16: 2946–2957.
Martinsson-Ahlzen HS, Liberal V, Grunenfelder B, Chaves SR, Spruck CH, Reed SI . Cyclin-dependent kinase-associated proteins Cks1 and Cks2 are essential during early embryogenesis and for cell cycle progression in somatic cells. Mol Cell Biol 2008; 28: 5698–5709.
Wang JJ, Fang ZX, Ye HM, You P, Cai MJ, Duan HB et al. Clinical significance of overexpressed cyclin-dependent kinase subunits 1 and 2 in esophageal carcinoma. Dis Esophagus 2013; 26: 729–736.
Lee SW, Kang SB, Dee DS, Lee JU . Akt, and Cks1 are related with lymph node metastasis in gastric adenocarcinoma. Hepatogastroenterology 2013; 60: 932–937 [Epub ahead of print].
Martin-Ezquerra G, Salgado R, Toll A, Baro T, Mojal S, Yebenes M et al. CSC28 protein kinase regulatory subunit 1B (CKS1B) expression and genetic status analysis in oral squamous cell carcinoma. Histol Histopathol 2011; 26: 71–77.
Wang XC, Tian LL, Tian J, Wu HL, Meng AM . Overexpression of Cks1 is associated with poor survival by inhibiting apoptosis in breast cancer. J Cancer Res Clin Oncol 2009; 135: 1393–1401.
Liu Z, Fu Q, Lv J, Wang F, Ding K . Prognostic implication of p27Kip1, Skp2 and Cks1 expression in renal cell carcinoma: a tissue microarray study. J Exp Clin Cancer Res 2008; 27: 51.
Kawakami K, Enokida H, Tachiwada T, Nishiyama K, Seki N, Nakagawa M . Increased SKP2 and CKS1 gene expression contributes to the progression of human urothelial carcinoma. J Urol 2007; 178: 301–307.
Slotky M, Shapira M, Ben-Izhak O, Linn S, Futerman B, Tsalic M et al. The expression of the ubiquitin ligase subunit Cks1 in human breast cancer. Breast Cancer Res 2005; 7: R737–R744.
Shapira M, Ben-Izhak O, Linn S, Futerman B, Minkov I, Hershko DD . The prognostic impact of the ubiquitin ligase subunits Skp2 and Cks1 in colorectal carcinoma. Cancer 2005; 103: 1336–1346.
Masuda TA, Inoue H, Nishida K, Sonoda H, Yoshikawa Y, Kakeji Y et al. Cyclin-dependent kinase 1 gene expression is associated with poor prognosis in gastric carcinoma. Clin Cancer Res 2003; 9: 5693–5698.
Inui N, Kitagawa K, Miwa S, Hattori T, Chida K, Nakamura H et al. High expression of Cks1 in human non-small cell lung carcinomas. Biochem Biophys Res Commun 2003; 303: 978–984.
Yamamoto S, Tsuda H, Miyai K, Takano M, Tamai S, Matsubara O . Cumulative alterations of p27-related cell-cycle regulators in the development of endometriosis-associated ovarian clear cell adenocarcinoma. Histopathology 2010; 56: 740–749.
Keller UB, Old JB, Dorsey FC, Nilsson JA, MacLean KH, Chung L et al. Myc targets Cks1 to provoke the suppression of p27Kip1, proliferation, and lymphomagenesis. EMBO J 2007; 26: 2562–2574.
Bhatt KV, Hu R, Spofford LS, Aplin AE . Mutant B-RAF signaling and cyclin D1 regulate Cks1/S-phase kinase-associated protein 2-mediated degradation of p27Kip1 in human melanoma cells. Oncogene 2007; 26: 1056–1066.
Shen DY, Zhan YH, Wang QM, Rui G, Zhang ZM . Oncogenic potential of cyclin kinase subunit-2 in cholangiocarcinoma. Liver Int 2013; 33: 137–148.
Perez-Magan E, Campos-Martin Y, Mur P, Fiano C, Ribalta T, Garcia JF et al. Genetic alterations associated with progression and recurrence in meningiomas. J Neuropathol Exp Neurol 2012; 71: 882–893.
Menghi F, Orzan FN, Eoli M, Farinotti M, Maderna E, Pisati F et al. DNA microarray analysis identifies CKS2 and LEPR as potential markers of meningioma recurrence. Oncologist 2011; 16: 1440–1450.
Chen R, Feng C, Xu Y . Cyclin-dependent kinase-associated protein Cks2 is associated with bladder cancer progression. J Int Med Res 2011; 39: 533–540.
Tanaka F, Matsuzaki S, Mimori K, Kita Y, Inoue H, Mori M . Clinicopathological and biological significance of CDC28 protein kinase regulatory subunit 2 overexpression in human gastric cancer. Int J Oncol 2011; 39: 361–372.
Wiese AH, Auer J, Lassmann S, Nahrig J, Rosenberg R, Hofler H et al. Identification of gene signatures for invasive colorectal tumor cells. Cancer Detect Prev 2007; 31: 282–295.
Lin HM, Chatterjee A, Lin YH, Anjomshoaa A, Fukuzawa R, McCall JL et al. Genome wide expression profiling identifies genes associated with colorectal liver metastasis. Oncol Rep 2007; 17: 1541–1549.
Li M, Lin YM, Hasegawa S, Shimokawa T, Murata K, Kameyama M et al. Genes associated with liver metastasis of colon cancer, identified by genome-wide cDNA microarray. Int J Oncol 2004; 24: 305–312.
Kawakami K, Enokida H, Tachiwada T, Gotanda T, Tsuneyoshi K, Kubo H et al. Identification of differentially expressed genes in human bladder cancer through genome-wide gene expression profiling. Oncol Rep 2006; 16: 521–531.
van’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002; 415: 530–536.
Liberal V, Martinsson-Ahlzen HS, Liberal J, Spruck CH, Widschwendter M, McGowan CH et al. Cyclin-dependent kinase subunit (Cks) 1 or Cks2 overexpression overrides the DNA damage response barrier triggered by activated oncoproteins. Proc Natl Acad Sci USA 2012; 109: 2754–2759.
Bourne Y, Watson MH, Hickey MJ, Holmes W, Rocque W, Reed SI et al. Crystal structure and mutational analysis of the human CDK2 kinase complex with cell cycle-regulatory protein CksHs1. Cell 1996; 84: 863–874.
Krude T . Mimosine arrests proliferating human cells before onset of DNA replication in a dose-dependent manner. Exp Cell Res 1999; 247: 148–159.
Bartek J, Lukas C, Lukas J . Checking on DNA damage in S phase. Nat Rev Mol Cell Biol 2004; 5: 792–804.
Wang CX, Tian J, Tian LL, Wu HL, Meng AM, Ma TH et al. Role of Cks1 amplification and overexpression in breast cancer. Biochem Biophys Res Commun 2009; 379: 1107–1113.
Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M . Control of SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature 2004; 428: 190–193.
Kunz C, Focke F, Saito Y, Schuermann D, Lettieri T, Selfridge J et al. Base excision by thymine DNA glycosylase mediates DNA-directed cytotoxicity of 5-fluorouracil. PLoS Biol 2009; 7: e1000091.
Acknowledgements
We wish to thank C Torres and Y Altman (Sanford-Burnham Medical Research Institute) for technical assistance with flow cytometry analyses. This work was supported by grants from the American Cancer Society (RSG-06-020-01) and National Institutes of Health (7R01HD049539-05) to CS, Department of Defense Breast Cancer Research Program (W81XWH-06-1-0344) to SVdR, and National Cancer Institute (CA074224) to SIR. Additional support came from a UCLH/UCL Comprehensive Biomedical Research Centre grant and was undertaken at UCLH/UCL, which received a portion of its funding from the Department of Health NIHR Biomedical Research Centres funding scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
del Rincón, S., Widschwendter, M., Sun, D. et al. Cks overexpression enhances chemotherapeutic efficacy by overriding DNA damage checkpoints. Oncogene 34, 1961–1967 (2015). https://doi.org/10.1038/onc.2014.137
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.137
This article is cited by
-
Increased expression of Cks1 protein is associated with lymph node metastasis and poor prognosis in nasopharyngeal carcinoma
Diagnostic Pathology (2017)
-
ADAM12-L confers acquired 5-fluorouracil resistance in breast cancer cells
Scientific Reports (2017)