Abstract
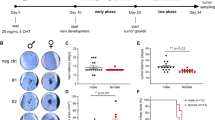
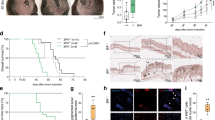
Phosphoinositide-dependent kinase-1 (PDK1) is a serine/threonine protein kinase that phosphorylates members of the conserved AGC kinase superfamily, including AKT and protein kinase C (PKC), and is implicated in important cellular processes including survival, metabolism and tumorigenesis. In large cohorts of nevi and melanoma samples, PDK1 expression was significantly higher in primary melanoma, compared with nevi, and was further increased in metastatic melanoma. PDK1 expression suffices for its activity, owing to auto-activation, or elevated phosphorylation by phosphoinositide 3′-OH-kinase (PI3K). Selective inactivation of Pdk1 in the melanocytes of BrafV600E::Pten–/– or BrafV600E::Cdkn2a–/–::Pten–/– mice delayed the development of pigmented lesions and melanoma induced by systemic or local administration of 4-hydroxytamoxifen. Melanoma invasion and metastasis were significantly reduced or completely prevented by Pdk1 deletion. Administration of the PDK1 inhibitor GSK2334470 (PDKi) effectively delayed melanomagenesis and metastasis in BrafV600E::Pten–/– mice. Pdk1–/– melanomas exhibit a marked decrease in the activity of AKT, P70S6K and PKC. Notably, PDKi was as effective in inhibiting AGC kinases and colony forming efficiency of melanoma with Pten wild-type (WT) genotypes. Gene expression analyses identified Pdk1-dependent changes in FOXO3a-regulated genes, and inhibition of FOXO3a restored proliferation and colony formation of Pdk1–/– melanoma cells. Our studies provide direct genetic evidence for the importance of PDK1, in part through FOXO3a-dependent pathway, in melanoma development and progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Choucair KA, Guerard KP, Ejdelman J, Chevalier S, Yoshimoto M, Scarlata E et al. The 16p13.3 (PDPK1) genomic gain in prostate cancer: a potential role in disease progression. Transl oncol 2012; 5: 453–460.
Shen H, Zhu Y, Wu YJ, Qiu HR, Shu YQ . Genomic alterations in lung adenocarcinomas detected by multicolor fluorescence in situ hybridization and comparative genomic hybridization. Cancer genetics and cytogenetics 2008; 181: 100–107.
Casamayor A, Morrice NA, Alessi DR . Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochemi J 1999; 342 (Pt 2): 287–292.
Maurer M, Su T, Saal LH, Koujak S, Hopkins BD, Barkley CR et al. 3-Phosphoinositide-dependent kinase 1 potentiates upstream lesions on the phosphatidylinositol 3-kinase pathway in breast carcinoma. Cancer Res 2009; 69: 6299–6306.
Finlay DK, Sinclair LV, Feijoo C, Waugh CM, Hagenbeek TJ, Spits H et al. Phosphoinositide-dependent kinase 1 controls migration and malignant transformation but not cell growth and proliferation in PTEN-null lymphocytes. J Exp Med 2009; 206: 2441–2454.
Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell 2009; 16: 21–32.
Bayascas JR, Leslie NR, Parsons R, Fleming S, Alessi DR . Hypomorphic mutation of PDK1 suppresses tumorigenesis in PTEN(+/−) mice. Curr Biol: CB 2005; 15: 1839–1846.
Miao B, Skidan I, Yang J, Lugovskoy A, Reibarkh M, Long K et al. Small molecule inhibition of phosphatidylinositol-3,4,5-triphosphate (PIP3) binding to pleckstrin homology domains. Proc Natl Acad Sci USA 2010; 107: 20126–20131.
Nagashima K, Shumway SD, Sathyanarayanan S, Chen AH, Dolinski B, Xu Y et al. Genetic and pharmacological inhibition of PDK1 in cancer cells: characterization of a selective allosteric kinase inhibitor. J Biol Chem 2011; 286: 6433–6448.
Eser S, Reiff N, Messer M, Seidler B, Gottschalk K, Dobler M et al. Selective requirement of PI3K/PDK1 signaling for Kras oncogene-driven pancreatic cell plasticity and cancer. Cancer cell 2013; 23: 406–420.
Bagrodia S, Smeal T, Abraham RT . Mechanisms of intrinsic and acquired resistance to kinase-targeted therapies. Pigment Cell Melanoma Res 2012; 25: 819–831.
Vultur A, Herlyn M . Cracking the system: melanoma complexity demands new therapeutic approaches. Pigment Cell Melanoma Res 2009; 22: 4–5.
Tsao H, Chin L, Garraway LA, Fisher DE . Melanoma: from mutations to medicine. Genes Dev 2012; 26: 1131–1155.
Lopez-Bergami P, Habelhah H, Bhoumik A, Zhang W, Wang LH, Ronai Z . RACK1 mediates activation of JNK by protein kinase C [corrected]. Mol Cell 2005; 19: 309–320.
Lopez-Bergami P, Huang C, Goydos JS, Yip D, Bar-Eli M, Herlyn M et al. Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell 2007; 11: 447–460.
Lopez-Bergami P, Kim H, Dewing A, Goydos J, Aaronson S, Ronai Z . c-Jun regulates phosphoinositide-dependent kinase 1 transcription: implication for Akt and protein kinase C activities and melanoma tumorigenesis. J Biol Chem 2010; 285: 903–913.
Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE Jr. et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genetics 2009; 41: 544–552.
Bosenberg M, Muthusamy V, Curley DP, Wang Z, Hobbs C, Nelson B et al. Characterization of melanocyte-specific inducible Cre recombinase transgenic mice. Genesis 2006; 44: 262–267.
Mora A, Komander D, van Aalten DM, PDK1 Alessi DR . the master regulator of AGC kinase signal transduction. Sem Cell Dev Biol 2004; 15: 161–170.
Bayascas JR . PDK1: the major transducer of PI 3-kinase actions. Cur Topics Microbiol Immunol 2010; 346: 9–29.
Smyth GK . Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 2004; 3: Article3.
You H, Jang Y, You-Ten AI, Okada H, Liepa J, Wakeham A et al. p53-dependent inhibition of FKHRL1 in response to DNA damage through protein kinase SGK1. Proc Natl Acad Sci USA 2004; 101: 14057–14062.
Huang H, Tindall DJ . Dynamic FoxO transcription factors. J Cell Sci 2007; 120: 2479–2487.
Najafov A, Sommer EM, Axten JM, Deyoung MP, Alessi DR . Characterization of GSK2334470, a novel and highly specific inhibitor of PDK1. Biochem J 2011; 433: 357–369.
Najafov A, Shpiro N, Alessi DR . Akt is efficiently activated by PIF-pocket- and PtdIns(3,4,5)P3-dependent mechanisms leading to resistance to PDK1 inhibitors. Biochem J 2012; 448: 285–295.
Axten JM, Grant SW, Heerding DA, Medina JR, Romeril SP, Tang J Chemical Compounds In: Office USP, (ed.). A1 ed. USA2011.
Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M et al. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem 2005; 280: 20814–20823.
Ellwood-Yen K, Keilhack H, Kunii K, Dolinski B, Connor Y, Hu K et al. PDK1 attenuation fails to prevent tumor formation in PTEN-deficient transgenic mouse models. Cancer Res 2011; 71: 3052–3065.
Gagliardi PA, di Blasio L, Orso F, Seano G, Sessa R, Taverna D et al. 3-phosphoinositide-dependent kinase 1 controls breast tumor growth in a kinase-dependent but akt-independent manner. Neoplasia 2012; 14: 719–731.
Aziz SA, Davies M, Pick E, Zito C, Jilaveanu L, Camp RL et al. Phosphatidylinositol-3-kinase as a therapeutic target in melanoma. Clin Cancer Res 2009; 15: 3029–3036.
Shi W, Oshlack A, Smyth GK . Optimizing the noise versus bias trade-off for Illumina whole genome expression BeadChips. Nucleic Acids Res 2010; 38: e204.
Benjamini Y, Hochberg Y . Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc Series B, Stat Methodol 1995; 57: 289–300.
Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 2004; 5: R80.
Medina JR, Becker CJ, Blackledge CW, Duquenne C, Feng Y, Grant SW et al. Structure-based design of potent and selective 3-phosphoinositide-dependent kinase-1 (PDK1) inhibitors. J Med Chem 2011; 54: 1871–1895.
Lawlor MA, Mora A, Ashby PR, Williams MR, Murray-Tait V, Malone L et al. Essential role of PDK1 in regulating cell size and development in mice. EMBO J 2002; 21: 3728–3738.
Acknowledgements
This manuscript is dedicated in fond memory to Victor Fung, Ph.D., a former Program Officer at NCI and a former Scientific Review Officer of the Cancer Etiology study section of CSR, NIH, for his wisdom, compassion, integrity, his love of the sciences and the arts and his contributions to the career development of so many investigators during his own distinguished career. We thank Dario Alessi for providing the conditional Pdk1–/– mice, Vincent Hearing for the Tyrp1 antibody and Pedro Aza-Blanc for small interfering RNA library work. Support by NIH grants CA099961 (to ZR) and CA128814 (to ZR and MP) and a Melanoma Research Alliance grant (to ZR) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Scortegagna, M., Ruller, C., Feng, Y. et al. Genetic inactivation or pharmacological inhibition of Pdk1 delays development and inhibits metastasis of BrafV600E::Pten–/– melanoma. Oncogene 33, 4330–4339 (2014). https://doi.org/10.1038/onc.2013.383
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.383
Keywords
This article is cited by
-
TMEM116 is required for lung cancer cell motility and metastasis through PDK1 signaling pathway
Cell Death & Disease (2021)
-
Chromatin remodellers Brg1 and Bptf are required for normal gene expression and progression of oncogenic Braf-driven mouse melanoma
Cell Death & Differentiation (2020)
-
STAT3 enhances the constitutive activity of AGC kinases in melanoma by transactivating PDK1
Cell & Bioscience (2019)
-
Melanocytic nevi and melanoma: unraveling a complex relationship
Oncogene (2017)
-
PDK1 promotes tumor growth and metastasis in a spontaneous breast cancer model
Oncogene (2016)