Abstract
p53 is one of the most studied genes in cancer biology, and mutations in this gene may be predictive for the development of many types of cancer in humans and in animals. However, whether p53 mutations in non-tumor stromal cells can affect tumor development has received very little attention. In this study, we show that B16F0 melanoma cells form much larger tumors in p53-deficient mice than in wild-type mice, indicating a potential role of p53 deficiency in non-tumor cells of the microenvironment. As mesenchymal stem cells (MSCs) are attracted to tumors and form a major component of the tumor microenvironment, we examined the potential role of p53 status in MSCs in tumor development. We found that larger tumors resulted when B16F0 melanoma cells were co-injected with bone marrow MSCs derived from p53-deficient mice rather than MSCs from wild-type mice. Interestingly, this tumor-promoting effect by p53-deficient MSCs was not observed in non-obese diabetic/severe combined immunodeficiency mice, indicating the immune response has a critical role. Indeed, in the presence of inflammatory cytokines, p53-deficient MSCs expressed more inducible nitric oxide synthase (iNOS) and exhibited greater immunosuppressive capacity. Importantly, tumor promotion by p53-deficient MSCs was abolished by administration of S-methylisothiourea, an iNOS inhibitor. Therefore, our data demonstrate that p53 status in tumor stromal cells has a key role in tumor development by modulating immune responses.
Similar content being viewed by others
Introduction
p53 is one of the most highly studied tumor-suppressor genes.1, 2, 3, 4 It is also one of the most frequently mutated genes associated with cancer. Indeed, p53 mutations can be detected in about 50% of all human tumors,5 including melanoma, ovarian carcinoma, liver cancer, colon cancer, lymphoma and leukemia.6, 7, 8, 9 It has been shown that p53 can activate a variety of genes related to tumor suppression,10 autoimmunity11, 12 and even maternal reproduction.13 Many key upstream regulators and downstream effectors of p53 that function in cell growth arrest have been described.14 Also, disruption of p53 was shown to revert chemotherapeutic agent-induced senescence and ultimately lead to tumor progression.15, 16 Recent studies have shown that p53 deficiency imparts survival advantages on tumor cells by inducing autophagy, a cell survival mechanism involving autonomous degradation of unnecessary or dysfunctional cellular components.17 p53 also has cytosolic activity that can induce apoptosis in a transcription-independent manner.18
In solid tumors, the tumor mass is composed of many different types of cells. Apart from the tumor cells themselves, the tumor stroma provides fertile soil for tumor growth.19 Mesenchymal stem cells (MSCs) are one of the major components of tumor stroma.20 These bone marrow-derived cells migrate to the tumor site where they form part of the microenvironment, and can affect tumor survival and angiogenesis even at the earliest stage of tumor development.21, 22 MSCs are multipotent cells and can be isolated from many different tissues.23 Our previous studies have shown that tumor-infiltrating MSCs dramatically enhance tumor growth by secreting CCR2 ligands and recruiting monocytes and macrophages.24 Interestingly, some studies have observed p53 mutations in the stromal cells associated with human tumors, such as colorectal cancer, bladder carcinoma and breast cancer.25, 26, 27, 28 However, whether mutated tumor-suppressor genes in stromal cells affect tumor progression has not been determined.
In addition to their differentiation potential, MSCs can also become strongly immunomodulatory. We have shown that mouse MSCs can potently suppress immune responses through the concerted action of chemokines and nitric oxide (NO).29, 30 NO, derived from L-arginine through the enzymatic activity of inducible NO synthase (iNOS), has been reported to affect various inter- and intracellular signaling pathways, including induction of T-cell apoptosis31 and inhibition of T-cell proliferation and cytokine production.32 Furthermore, MSCs have the potential to affect the function and migration of other immune cells, including macrophages, dendritic cells and B cells.23 Taken together, these findings indicate that MSCs are critical for modulating the immune response at tumor sites. Less clear, however, is whether tumor-suppressor gene status in stromal cells like MSCs influences the growth and development of the tumor; this interaction is poorly defined and remains largely controversial.33, 34, 35, 36 A better understanding of this relationship would likely provide important insights into tumor development. Therefore, we investigated the role of tumor-suppressor gene status in tumor stromal cells.
To examine the contribution of p53 status in non-cancer cells to tumor development, we administered melanoma cells into syngeneic p53-deficient mice and wild-type controls, and found significantly larger tumors in p53-deficient mice. This was reproduced by co-administration of p53-deficient MSCs and melanoma cells into wild-type mice, and was therefore wholly dependent on MSCs. This tumor-promoting capacity of p53-deficient MSCs is related to their immunoregulatory effects because it was not observed in similarly treated immunodeficient mice. Importantly, we found that genetic deficiency or knockdown of p53 in MSCs resulted in dramatic increases in iNOS expression, NO production and immunosuppressive potential. Moreover, an iNOS inhibitor completely eliminated the tumor-promoting effect of p53−/− MSCs. We conclude that p53 deficiency in MSCs contributes significantly to tumor development by regulating microenvironment immune responses in an iNOS-dependent manner. Our study reveals a critical role for p53 status in MSCs during tumor progression, a finding with potential implications for antitumor therapy.
Results
Melanoma cells form larger tumors in p53-deficient mice
Studies of tumor-suppressor genes in cancer have focused mainly on their role in the tumor cells themselves, while largely ignoring their status in non-tumor cells. The role of tumor-suppressor genes in non-tumor cells deserves greater attention because large numbers of cells in most tumor masses are not tumor cells per se. Mutations in p53 are associated with the development of many types of tumor.37, 38, 39 We investigated the influence of p53 status in non-tumor cells on tumor growth. C57BL/6 mice with the following genotypes: p53+/+, p53+/− or p53−/−, were injected with B16F0 cells, a melanoma cell line that originated in C57BL/6 mice. We found that much larger melanoma formed in p53−/− mice, as compared with p53+/+ and p53+/− littermate controls (Figure 1a), suggesting that p53 ablation in cells other than tumor cells contributes significantly to tumor development. Therefore, p53 not only affects the tumor cells themselves, but also affects tumors by modulating cells of the tumor microenvironment.
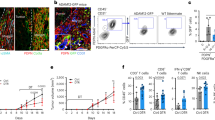
B16 melanoma growth is dependent on MSCs in the tumor microenvironment. (a) B16F0 melanoma cells (4 × 105) were administered into p53+/+, p53+/− or p53−/− mice (n=6 each) intramuscularly. After 3 weeks, tumors were excised and weighed. (b) A large proportion of tumor-associated MSCs originated from bone marrow cells. Lethally irradiated C57BL/6 mice were transplanted with bone marrow cells from green fluorescent protein (GFP)-transgenic C57BL/6 mice via intra-bone injection. Three months later, these mice were injected with EL4 lymphoma cells (5 × 105 cells). MSCs were isolated from the tumors and cultured for 12 days. Microscopic image of the same field were taken under bright field and ultraviolet-light illumination to reveal GFP-expressing cells. Scale bar=100 μm.
MSCs are essential for tumor promotion in a p53-deficient background
MSCs are known to be recruited to sites of damaged tissue. As tumor is considered to be a wound that never heals,19 MSCs would be expected to migrate to tumors as well. To examine whether MSCs are indeed recruited into tumors, we performed intra-bone marrow transplantation of bone marrow from green fluorescent protein-transgenic C57BL/6 mice into lethally irradiated syngeneic mice. Three months later, tumor cells were injected into these mice. The resultant tumors were excised and MSCs isolated to examine for the presence of engrafted MSCs by their green fluorescence. We found that a large percentage of tumor-infiltrated MSCs expressed green fluorescent protein (Figure 1b), indicating the successful engraftment and migration of the transplanted MSCs. As MSCs are highly resistant to radiation, many of the host’s MSCs would be expected to survive radiation ablation. Therefore, the detection of even a few engrafted MSCs is significant.
MSCs are a major component of the tumor microenvironment and are believed to have a significant role in tumor growth.22, 40 We hypothesized that p53 deficiency in MSCs in the tumor microenvironment contributes to tumor development. To test this hypothesis, MSCs were isolated from p53-deficient mice and wild-type controls according to well-established protocols.28, 41, 42 These cells expressed the typical MSC phenotype: CD34−CD11b−CD11c−CD45−MHC class II−CD44+Sca-1+MHC class Ilow, and were able to differentiate into adipocytes and osteoblasts under the appropriate culture conditions. To test their effect on tumor development, p53+/+ or p53−/− MSCs were co-administered along with B16F0 cells into syngeneic wild-type mice. We found that exogenous p53−/− MSCs dramatically increased tumor growth, to almost the same levels that occurred in p53−/− mice, whereas p53+/+ MSCs had little effect (Figure 2a). To test whether this effect also exists in other tumor types, we co-injected EL4 lymphoma cells along with p53+/+ or p53−/− MSCs into syngeneic mice. We found that p53-deficient MSCs also significantly promoted tumor growth in this EL4 lymphoma model (Figure 2b), demonstrating that the tumor-promoting effect of p53-deficient MSCs is not tumor type specific. Therefore, these experiments showed that the tumor-promoting effect of p53 deficiency in non-tumor cells could be largely contributed by MSCs.
B16 melanoma growth promotion by p53-deficient MSCs is exerted by modulating the immune response. (a) B16F0 melanoma cells (4 × 105) were co-injected with p53+/+ or p53−/− MSCs (2 × 105) intramuscularly into C57BL/6 mice; control group received B16F0 cells alone (n=6 each). Tumors were excised after 10 days and weighed. (b) Same tumor progression assay as described in (a) using EL4 lymphoma cells (n=6 each). Tumors were excised after 2 weeks and weighed. (c) B16F0 melanoma cells were co-cultured with conditioned medium of p53+/+ or p53−/− MSCs. Cells were trypsinized and counted using a hemacytometer on the indicated days. (d) Same tumor progression assay as described in (a) was performed in NOD/SCID mice (n=8 each). Tumors were excised after 12 days and weighed. Tumor weights are shown as means±s.e.m.
The tumor-promoting effect of p53-deficient MSCs is exerted through the immune system
Our experiments clearly demonstrate that p53 deficiency in MSCs allows them to significantly promote tumor development. There are two possible mechanisms to account for this effect in vivo: a direct effect on tumor cell growth, or an indirect effect through modulation of the host immune response to tumor. To test the former, we co-cultured B16F0 cells in the presence or absence of conditioned medium from p53+/+ or p53−/− MSCs, but found no effect on tumor growth (Figure 2c). Therefore, p53-deficient MSCs do not affect tumor cell growth directly. We next tested for an indirect effect.
Previous studies have shown that the immune response has a critical role in modulating tumor development.43, 44 Therefore, to investigate whether the immune system is also involved in the effects of p53 status in MSCs on tumor development, we utilized immunodeficient non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice. NOD/SCID mice and wild-type mice were injected with B16F0, alone or with p53+/+ or p53−/− MSCs. As in the experiments above, p53−/− MSCs again significantly promoted tumor growth in wild-type mice. However, the effects of co-administration of p53-deficient MSCs were not significant in NOD/SCID mice (Figure 2d). Therefore, the tumor-promoting effect of p53-deficient MSCs must be exerted indirectly through the immune system.
p53 modulates the immunosuppressive activity of MSCs
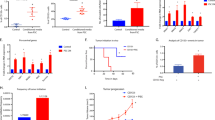
To test whether the tumor-promoting effect of p53 status in MSCs is indeed exerted through modulation of the immune system, we co-cultured p53−/− or wild-type MSCs with activated splenocytes in vitro, as we have previously performed with wild-type MSCs.29 Splenocytes isolated from wild-type mice were stimulated with anti-CD3 and anti-CD28, a well-established protocol to stimulate T cells. To systematically compare the effects of wild-type MSCs and p53−/− MSCs, graded numbers of MSCs were added to a fixed number of splenocytes, and resultant splenocyte proliferation was assayed by [3H] thymidine incorporation. At high MSC-to-splenocyte ratios, MSCs derived from either p53+/+ or p53−/− mice completely suppressed T-cell proliferation, as we previously reported.29 However, at low ratios, such as 1:80 or 1:160 (MSC-to-splenocyte), p53−/− MSCs were much more potent inhibitors of T-cell proliferation (Figure 3a). Therefore, MSCs with p53 deficiency have significantly enhanced immunosuppressive capacity.
p53 deficiency promotes the immunosuppressive effect of MSCs in vitro. Fresh C57BL/6 splenocytes were co-cultured with p53+/+ or p53−/− MSCs and stimulated with anti-CD3 and anti-CD28 (1.74 μg/ml each). Various MSC-to-splenocyte ratios were used in co-culture, as indicated. (a) Proliferation after 48 h was assayed by 3H-Tdr incorporation. (b) NG-monomethyl-L-arginine acetate salt (L-NMMA; 1 mM), an iNOS inhibitor, was added to the co-culture system, at MSC-to-splenocyte ratios of 1:20 (bottom left panel) or 1:160 (bottom right panel), respectively. The extent of cell aggregation was observed microscopically after 48 h. Scale bar=100 μm. Data are shown as means±s.e.m. from a representative of three experiments.
Our previous studies have shown that murine MSCs primed by proinflammatory cytokines exert their immunosuppressive function by producing large amounts of NO.29 Therefore, we next examined whether the enhanced immunosuppressive capacity of p53−/− MSCs results from yet more abundant NO production. The MSC-to-splenocyte co-culture assay was performed at 1:20 and 1:160 ratios (MSC-to-splenocyte) using p53+/+ or p53−/− MSCs in the presence or absence of the iNOS inhibitor, NG-monomethyl-L-arginine acetate salt. We found that iNOS inhibition completely reversed p53+/+ or p53−/− MSC-mediated suppression of splenocyte proliferation, as evidenced by proliferation assay and cluster formation observed by microscopy (Figure 3b). Therefore, the exaggerated immunosuppression by p53-deficient MSCs is also dependent solely on NO.
p53−/− MSCs express higher iNOS and produce greater amounts of NO upon stimulation by proinflammatory cytokines
The immunosuppressive capability of mouse MSCs is dependent on the induction of iNOS expression by proinflammatory cytokines. MSCs from iNOS-deficient mice are ineffective in generating immunosuppression.29, 30 To determine whether p53 affects iNOS induction in MSCs, we stimulated wild-type or p53−/− MSCs with interferon γ (IFNγ) and tumor necrosis factor α (TNFα; 10 ng/ml each). p53−/− MSCs showed a striking increase in iNOS, in terms of mRNA and protein (Figures 4a and b), much higher than that occurred with p53+/+ or p53+/− MSCs. As expected, when NO in the supernatant was quantified by Griess reagent, we found that p53−/− MSCs produced more than twice as much NO than did their wild-type counterparts (Figure 4c). Therefore, p53 must inhibit inflammatory cytokine-induced iNOS expression in MSCs, such that p53 deficiency results in still greater induction of iNOS expression and thereby enhanced NO production.
p53-deficient MSCs produce more NO than wild-type MSCs on stimulation with inflammatory cytokine. MSCs derived from p53+/+, p53+/− or p53−/− mice were stimulated with IFNγ plus TNFα (10 ng/ml each). (a) After cultured for 4, 8, 12 or 24 h, MSCs were assayed for iNOS mRNA expression by real-time PCR, and compared with β-actin expression. (b) Protein levels of iNOS in MSCs cultured for 7.5 or 24 h were determined by western blotting analysis. The density of protein bands was measured using Quantity One (Bio-Rad, Hercules, CA, USA) and shown as iNOS/β-actin. (c) After 24 h or 48 h culture, NO in supernatant was assayed as total nitrate using a modified Griess reagent. Nitrate values are shown as means±s.e.m. of three wells from a representative of three experiments.
One caveat with using MSCs derived from p53-deficient mice is that the changes in MSCs could be secondary to a developmental effect of p53 deficiency. To further verify the direct role of p53 in MSCs, we used short hairpin RNA to knockdown p53 expression in isolated wild-type MSCs. This approach effectively knockdown p53 in MSCs as demonstrated by the mRNA level using quantitative PCR (Figure 5a) and protein level using western blotting analysis (Figure 5b). Interestingly, we found that iNOS mRNA expression in these p53 knockdown cells dramatically increased on treatment with IFNγ and TNFα (Figure 5c), as compared with cells transfected with control sequence. NO production was similarly enhanced in p53 knockdown cells (Figure 5d). This result rules out any secondary development effect of p53 deficiency, and further verifies the effect of p53 on iNOS expression and NO production by MSCs.
p53 knockdown in MSCs increases production of NO. MSCs were transfected with p53 short hairpin RNA or control sequence during a 24-h incubation and followed by a puromycin (3 μg/ml) selection. Knockdown efficiency was determined by real-time PCR (a) and western blotting analysis (b). Cells were treated with or without IFNγ and TNFα (10 ng/ml each) for 8 h (a) and 24 h (b). iNOS mRNA level and total nitrate were assayed after a 24-h stimulation by real-time PCR (c) and Griess assay (d) respectively. Nitrate concentrations are shown as means±s.e.m.
Critical role of NO in tumor growth promotion by p53−/− MSCs in vivo
Using our in vitro system, we have clearly demonstrated that p53 deficiency boosts iNOS expression and leads to greater NO production. It is unclear, however, whether tumor growth enhancement by p53−/− MSCs is actually related to the observed increase in iNOS. To investigate the role of iNOS in vivo, an iNOS inhibitor, S-methylisothiourea hemisulfate salt (SMT), was utilized to block NO production. B16F0 melanoma cells were co-injected with p53+/+ or p53−/− MSCs intramuscularly into syngeneic mice. SMT (500 μg per mouse) or vehicle control was injected intraperitoneally everyday starting from the second day after tumor inoculation. Without SMT, B16F0 melanoma grew much more robustly with co-injection of p53−/− MSCs, as described above. In contrast, SMT administration completely reversed this effect (Figure 6), indicating that p53 deficiency in MSCs must promote tumor growth by radically enhancing the expression of iNOS, and subsequently suppressing the antitumor immune response.
Tumor promotion by p53-deficient MSCs in vivo is iNOS-dependent. B16F0 melanoma cells (4 × 105) were co-injected with p53+/+ or p53−/− MSCs (2 × 105) intramuscularly into C57BL/6 mice (n=6 each); controls were not injected with MSCs. SMT, an iNOS inhibitor, or vehicle (Ctrl) was administrated daily (500 μg per mouse) starting on day 2 post-tumor inoculation. In all groups, tumors were excised after 2 weeks and weighed. Tumor weights are shown as means±s.e.m.
Discussion
Most current challenges in cancer therapy are not the debulking of solid tumor through surgical resection, radiation therapy or chemotherapy, but the almost inevitable and lethal recurrence of many types of tumors.45 Stromal stem cells around the tumor, which are more resistant to standard treatments, may explain this recurrence.19, 43 Most studies of tumor stromal cells have focused on their ability to produce tumor-supportive molecules. Very few studies have examined genetic aberration in these non-tumor cells, which does occur under certain selective pressures during multiple cell interactions.46, 47, 48 To examine whether genetic alterations in stromal cells are capable of affecting tumor growth, we administered tumor cells into wild-type and p53−/− mice and found that indeed larger tumors resulted in p53−/− mice. The abundance of MSCs within tumor led us to explore their role in the tumor microenvironment. Notably, p53-deficient MSCs dramatically promoted tumor growth compared with wild-type MSCs. Furthermore, this tumor-promoting effect was diminished in immunodeficient mice, suggesting the involvement of the immune response. We showed that p53-deficient MSCs produce greater levels of NO, and result in more vigorous immunosuppression. Moreover, inhibition of iNOS was found to reverse the enhanced tumor promotion by p53-deficient MSCs. Therefore, p53 mutations in MSCs of the tumor stroma can promote tumor development through downregulation of antitumor immune responses.
Tumors have a complicated architecture, composed of many other cells besides the tumor cells themselves. In our studies, even the same B16F0 melanoma cells grown in mice with different p53 status could lead to remarkably different outcomes. p53 deficiency in cells other than tumor cells resulted in enhanced tumor growth, compared with a normal microenvironment, strongly suggesting a critical role of the tumor microenvironment in tumor development. Increasingly, reports have focused on several aspects of the tumor stroma. Kuperwasser et al.49 used a xenograft model to prove that gene modifications in stromal cells before co-implantation of normal epithelial cells could result in the outgrowth of malignant lesions. Recent studies also showed that two oncogenic miRNAs, miR-17-92a and miR-106b-25, were upregulated in colorectal cancer stroma, suggesting that miRNAs in cancer stroma are probably involved in tumor progression.50 Furthermore, several studies have demonstrated MSC tropism on tumors, and our recent work also proved that MSCs can recruit macrophages and promote tumor growth.24, 51, 52 Emerging evidence supports the idea that induction of mutations in stromal cells can promote tumor formation. Genetic mutations in stromal cells have been reported in breast cancer.28, 46 Maffini et al.53 demonstrated that when the stroma, but not epithelial cells, was exposed to chemical carcinogen they resulted in neoplastic transformation of the mammary epithelial cells. Thus, we hypothesized that MSCs have a critical role in tumor promotion, and then demonstrated a tumor-promoting effect by p53−/− MSCs co-injected with tumor cells. Hence, stromal cells are an indispensable component in tumor development.
Our studies prove that T-cell function can be strongly inhibited by p53-deficient MSCs and thus tumor growth is unchecked. This result provides further evidence that abundant immune cells and inflammatory cytokines at tumor sites are requisite for tumor growth. In contrast, there are many reports of antitumor effects on tumor-infiltrating immune cells. Many studies have demonstrated that infiltrating T cells can be found in various cancers, including ovarian cancer, renal cell carcinoma and bladder cancer.54, 55, 56 The presence of CD3+ and CD8+ T cells has been shown to be closely related to a better prognosis.57 Vδ1+ T cells display antitumor activity in vitro and have been used to treat patients with metastatic melanoma.58 Furthermore, treatment with adoptive T-cell transfer can result in synergistic anti-neoplastic effects owing to the increased immunogenicity of cancer cells.59 Yet, for all these antitumor effects, there appears to be a balance between anti- and pro-tumor effects by both CD4+ and CD8+ T cells,60, 61, 62 and regulatory T cells can migrate into the microenvironment and suppress antitumor responses in human ovarian carcinoma.63 Considering the variable effects of this multitude of stromal cell and immune response interactions, the mechanism by which p53 deficiency in MSCs affects T-cell function in tumors warrants further exploration.
In addition to T-cell effects, tumor-infiltrating macrophages have been found to facilitate angiogenesis, matrix breakdown and tumor cell motility by expression of pro-angiogenic factors, such as vascular endothelial growth factor and proteases like urokinase-type plasminogen activator and matrix metalloproteinases that remodel the extracellular matrix.64 Our previous studies also showed that tumor-resident MSCs are involved in orchestrating tumor microenvironment effects through recruitment of monocytes/macrophages, thereby facilitate tumor growth.29, 30 Therefore, the effect of p53-deficient MSCs on tumor-resident macrophages also must be further investigated before we can complete the whole picture of the role of the tumor microenvironment in tumor growth.
We have demonstrated that p53 deficiency allows enhanced upregulation of iNOS expression and NO production in MSCs. NO has long been acknowledged as an important factor in tumor growth for its effects on antitumor immunity and possibly cell survival. However, whether NO directly promotes or blocks tumor growth remains controversial. It is claimed that p53−/−NOS2+/+ C57BL/6 mice, which display greater NO production, experience accelerated spontaneous tumor development, compared with p53−/−NOS2−/− mice.65 In contrast, increased iNOS expression was also reported to be cytotoxic for tumor cells, since accumulated NO would trigger p53-mediated growth arrest and apoptosis.66 With the guardian p53 absence, accumulated NO would impair T-cell function and, in turn, facilitate tumor progression. NO has been shown to exert suppressive effects on T cells at several levels. It has been reported that NO affects immune cells by damaging genomic DNA, mitochondrial respiratory chain and ribonucleotide reductase activity.67, 68, 69, 70 In addition, it has been reported that peroxynitrite, the products of NO, can inhibit T lymphocyte activation and proliferation by impairing tyrosine phosphorylation.71 Both tyrosine nitration and protein carbonylation can exert strong effects on immune cells.72, 73 Studies also showed that NO can disrupt the JAK3/STAT5 signaling pathway in T cells, resulting in decreased T-cell proliferation, however, tyrosine nitrosylation of Jak3/STAT5 was not changed.74 Nevertheless, high level of NO is well known to have significant effects on T cells.
In our studies, we found that high levels of NO production by p53-deficient MSCs had strong inhibitory effects on T cells, but no direct effects on tumor cell growth. However, other reports showed that murine melanoma cells transfected with iNOS showed reduced tumorigenicity and metastatic potential, proving the important role of NO in the antitumor effect.75 It is possible that the quantity of NO determines what effect it may exert on tumor; the potential equilibrium between pro- or antitumor effects needs further investigation. p53 can decrease iNOS expression by downregulating iNOS promoter activity in certain human tumor cell lines and murine fibroblasts.76 Yet, details of the mechanism by which p53 regulates iNOS expression in MSCs should be explored further.
In conclusion, the results presented here suggest the following scenario: changes in p53 status in tumor stromal cells lead to the facilitation of tumor growth through enhanced NO production and immunomodulation. Therefore, in addition to p53 deficiency in tumor cells themselves, the same defect in cells of the tumor microenvironment, especially MSCs, also supports tumor development. With further study and a more complete understanding of these multifaceted interactions, it may become possible to devise strategies that target tumor stromal cells as a novel mode of cancer therapy.
Materials and methods
Mice
C57BL/6 and NOD-SCID mice were purchased from the Shanghai Laboratory Animal Center of Chinese Academy of Sciences, Shanghai, China, and maintained under specific pathogen-free conditions. p53−/− mice were from Model Animal Research Center of Nanjing University, Nanjing, China. Mice were maintained in the vivarium of Shanghai Jiao Tong University School of Medicine, Shanghai, China. Animals were matched for age and gender in each experiment. All procedures were approved by the Institutional Animal Care and Use Committee of the Institute of Health Sciences, Shanghai Institutes for Biological Sciences of Chinese Academy of Sciences.
Cells
MSCs were generated from tibia and femur bone marrow or by isolation from compacted bone digested with type II collagenase (Sigma-Aldrich, St Louis, MO, USA) using 6- to 10-week-old mice. Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (all from Invitrogen, Carlsbad, CA, USA). Eight hours later, non-adherent cells were removed and adherent cells were maintained with medium replenishment every 3 days. Splenocytes (1 × 106 cells/ml) were activated by soluble anti-CD3 and anti-CD28 (1.74 μg/ml each) for 48 h. All T-cell cultures were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin.
Reagents
Recombinant mouse IFNγ and TNFα were from eBiosciences (La Jolla, CA, USA). NG-monomethyl-L-arginine acetate salt, SMT and puromycin were from Sigma-Aldrich. Mouse CD3 and CD28 polyclonal antibodies were from Abcam (Cambridge, MA, USA).
Proliferation assay
To assay cell proliferation, 0.5 μCi of 3H-thymidine (Tdr, Shanghai Institute of Applied physics, Chinese Academy of Sciences, China) was added to each well 4 to 6 h before termination of the cultures by freezing. Incorporated 3H-Tdr was assessed using a Wallac Microbeta scintillation counter (Perkin-Elmer, Waltham, MA, USA).
Real-time PCR
Total RNA was isolated using RNAprep pure Cell/Bacteria Kit (Tiangen Biotech, Beijing, China), and first-strand complementary DNA synthesis was performed using 1st cDNA Synthesization Kit with oligo(dT)15 (Tiangen Biotech, Beijing, China). The levels of mRNA of genes of interest were measured by real-time PCR (7900 HT by Applied Biosystems, Foster City, CA, USA) using SYBR Green Master Mix (Roche Diagnostics, Indianapolis, IN, USA). Total amount of mRNA was normalized to endogenous β-actin mRNA. Sequences of PCR primer pairs were as follows: mouse p53, forward 5′-GTCACAGCACATGACGGAGG-3′ and reverse 5′-TCTTCCAGATACTCGGGATAC-3′; mouse iNOS, forward 5′-CAGCTGGGCTGTACAAACCTT-3′ and reverse 5′-CATTGGAAGTGAAGCGTTTCG-3′; mouse β-actin, forward 5′-TTCCAGCCTTCCTTCTTGGG-3′ and reverse 5′-TGTTGGCATAGAGGTCTTTACGG-3′.
Detection of NO
NO was detected using a modified Griess reagent (Sigma-Aldrich). Briefly, all NO3− was converted into NO2− by nitrate reductase, and total NO2− was detected by the Griess reaction.77
Western blot
Protein samples in sodium dodecyl sulfate sample buffer were heated at 95 °C for 10 min and separated on sodium dodecyl sulfate–polyacrylamide gels. Then proteins were electroblotted to polyvinylidene difluoride membranes and revealed by mouse and rabbit antibodies against p53, iNOS or β-actin by overnight incubation at 4 °C. The antibodies were as follows: anti-mouse iNOS and anti-mouse p53 (Cell Signaling Technology, Inc., Shanghai, China); anti-mouse β-actin (Sigma, St Louis, MO, USA). After three washes with tris-buffered saline and tween20, membranes were incubated with anti-mouse or anti-rabbit secondary antibodies (Cell Signaling Technology, Inc.). Finally, the blot was subjected to chemiluminescent detection according to the manufacturer’s instructions.
Lentiviral infections
Mouse p53 short hairpin RNA lentiviral particles were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Particles were added to MSCs cultures 1 day before puromycin screening. After infection, cells were selected using 3 μg/ml puromycin for 3 days and used for the following experiments.
Mouse tumor model
B16F0 mouse melanoma cells or EL4 lymphoma cells were expanded in complete Dulbecco’s modified Eagle’s medium in vitro. Each mouse was injected with B16F0 cells or EL4 cells (4 × 105 in 100 μl phosphate-buffered saline) intramuscularly on the left thigh, with or without co-injection of different types of MSCs (2 × 105 cells). SMT was administered intraperitoneally at 500 μg per day per mouse starting on day 2 after tumor inoculation. Mice were observed daily and killed when tumor burden began to significantly affect mobility. The tumors were then excised and weighed.
Statistical analysis
Data are presented as mean±s.e.m. Statistical significance was assessed by unpaired two-tailed Student’s t-test.
Abbreviations
- MSCs:
-
mesenchymal stem cells
- NO:
-
nitric oxide
- iNOS:
-
inducible nitric oxide synthase
- IFNγ:
-
interferon-γ
- TNFα:
-
tumor necrosis factor-α
- SMT:
-
S-methylisothiourea hemisulfate salt
References
Lane DP, Crawford LV . T antigen is bound to a host protein in SV40-transformed cells. Nature 1979; 278: 261–263.
Linzer DI, Levine AJ . Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell 1979; 17: 43–52.
Vogelstein B, Kinzler KW . p53 function and dysfunction. Cell 1992; 70: 523–526.
Baker S, Markowitz S, Fearon E, Willson J, Vogelstein B . Suppression of human colorectal carcinoma cell growth by wild-type p53. Science 1990; 249: 912–917.
Murray-Zmijewski F, Slee EA, Lu X . A complex barcode underlies the heterogeneous response of p53 to stress. Nat Rev Mol Cell Biol 2008; 9: 702–712.
Hollstein M, Sidransky D, Vogelstein B, Harris CC . P53 mutations in human cancers. Science 1991; 253: 49–53.
Hodis E, Watson I, Kryukov G, Arold S, Imielinski M, Theurillat J-P et al. A landscape of driver mutations in melanoma. Cell 2012; 150: 251–314.
Stretch J, Gatter K, Ralfkiaer E, Lane D, Harris A . Expression of mutant p53 in melanoma. Cancer Res 1991; 51: 5976–5985.
The Cancer Genome Atlas Research Network, Integrated genomic analyses of ovarian carcinoma. Nature 2011; 474: 609–615.
Zilfou JT, Lowe SW . Tumor suppressive functions of p53. Cold Spring Harb Perspect Biol 2009; 1: a001883.
Yamanishi Y, Boyle DL, Rosengren S, Green DR, Zvaifler NJ, Firestein GS . Regional analysis of p53 mutations in rheumatoid arthritis synovium. Proc Natl Acad Sci USA 2002; 99: 10025–10030.
Simelyte E, Rosengren S, Boyle DL, Corr M, Green DR, Firestein GS . Regulation of arthritis by p53: critical role of adaptive immunity. Arthritis Rheum 2005; 52: 1876–1884.
Levine AJ, Tomasini R, McKeon FD, Mak TW, Melino G . The p53 family: guardians of maternal reproduction. Nat Rev Mol Cell Biol 2011; 12: 259–265.
Giono LE, Manfredi JJ . The p53 tumor suppressor participates in multiple cell cycle checkpoints. J Cell Physiol 2006; 209: 13–20.
Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 2002; 109: 335–346.
Roninson IB . Tumor cell senescence in cancer treatment. Cancer Res 2003; 63: 2705–2715.
Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D'Amelio M et al. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol 2008; 10: 676–687.
Green DR, Kroemer G . Cytoplasmic functions of the tumour suppressor p53. Nature 2009; 458: 1127–1130.
Dvorak H . Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315: 1650–1659.
Joyce J, Pollard J . Microenvironmental regulation of metastasis. Nat Rev Cancer 2009; 9: 239–291.
Bergfeld S, DeClerck Y . Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev 2010; 29: 249–310.
Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M . Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res 2002; 62: 3603–3608.
Uccelli A, Moretta L, Pistoia V . Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008; 8: 726–762.
Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFalpha. Cell Stem Cell 2012; 11: 812–824.
Kurose K, Gilley K, Matsumoto S, Watson P, Zhou X-P, Eng C . Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet 2002; 32: 355–362.
Matsumoto N, Yoshida T, Yamashita K, Numata Y, Okayasu I . Possible alternative carcinogenesis pathway featuring microsatellite instability in colorectal cancer stroma. Br J Cancer 2003; 89: 707–719.
Paterson R, Ulbright T, MacLennan G, Zhang S, Pan C-X, Sweeney C et al. Molecular genetic alterations in the laser-capture-microdissected stroma adjacent to bladder carcinoma. Cancer 2003; 98: 1830–1836.
Patocs A, Zhang L, Xu Y, Weber F, Caldes T, Mutter G et al. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N Engl J Med 2007; 357: 2543–2551.
Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts A et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008; 2: 141–191.
Li W, Ren G, Huang Y, Su J, Han Y, Li J et al. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ 2012; 19: 1505–1513.
Bogdan C . Nitric oxide and the immune response. Nat Immunol 2001; 2: 907–916.
MacMicking J, Xie QW, Nathan C . Nitric oxide and macrophage function. Annu Rev Immunol 1997; 15: 323–350.
Addadi Y, Moskovits N, Granot D, Lozano G, Carmi Y, Apte R et al. p53 status in stromal fibroblasts modulates tumor growth in an SDF1-dependent manner. Cancer Res 2010; 70: 9650–9658.
Moskovits N, Kalinkovich A, Bar J, Lapidot T, Oren M . p53 Attenuates cancer cell migration and invasion through repression of SDF-1/CXCL12 expression in stromal fibroblasts. Cancer Res 2006; 66: 10671–10677.
Armesilla-Diaz A, Elvira G, Silva A . p53 regulates the proliferation, differentiation and spontaneous transformation of mesenchymal stem cells. Exp Cell Res 2009; 315: 3598–4208.
Rodriguez R, Rubio R, Masip M, Catalina P, Nieto A, de la Cueva T et al. Loss of p53 induces tumorigenesis in p21-deficient mesenchymal stem cells. Neoplasia (New York, NY) 2009; 11: 397–804.
Donehower L, Harvey M, Slagle B, McArthur M, Montgomery C, Butel J et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 1992; 356: 215–236.
Harvey M, Vogel H, Morris D, Bradley A, Bernstein A, Donehower L . A mutant p53 transgene accelerates tumour development in heterozygous but not nullizygous p53-deficient mice. Nat Genet 1995; 9: 305–316.
Lavigueur A, Maltby V, Mock D, Rossant J, Pawson T, Bernstein A . High incidence of lung, bone, and lymphoid tumors in transgenic mice overexpressing mutant alleles of the p53 oncogene. Mol Cell Biol 1989; 9: 3982–3991.
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007; 449: 557–563.
Soleimani M, Nadri S . A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc 2009; 4: 102–106.
Zhu H, Guo ZK, Jiang XX, Li H, Wang XY, Yao HY et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc 2010; 5: 550–560.
Coussens LM, Werb Z . Inflammation and cancer. Nature 2002; 420: 860–867.
Dougan M, Dranoff G . Immune therapy for cancer. Annu Rev Immunol 2009; 27: 83–117.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Moinfar F, Man YG, Arnould L, Bratthauer GL, Ratschek M, Tavassoli FA . Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res 2000; 60: 2562–2566.
Tuhkanen H, Anttila M, Kosma V-M, Ylä-Herttuala S, Heinonen S, Kuronen A et al. Genetic alterations in the peritumoral stromal cells of malignant and borderline epithelial ovarian tumors as indicated by allelic imbalance on chromosome 3p. Int J Cancer 2004; 109: 247–299.
Hill R, Song Y, Cardiff R, Van Dyke T . Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell 2005; 123: 1001–1012.
Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA 2004; 101: 4966–4971.
Nishida N, Nagahara M, Sato T, Mimori K, Sudo T, Tanaka F et al. Microarray analysis of colorectal cancer stromal tissue reveals upregulation of two oncogenic miRNA clusters. Clin Cancer Res 2012; 18: 3054–3070.
Zou W, Zheng H, He TC, Chang J, Fu YX, Fan W . LIGHT delivery to tumors by mesenchymal stem cells mobilizes an effective antitumor immune response. Cancer Res 2012; 72: 2980–2989.
Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 2009; 27: 2614–2623.
Maffini MV, Soto AM, Calabro JM, Ucci AA, Sonnenschein C . The stroma as a crucial target in rat mammary gland carcinogenesis. J Cell Sci 2004; 117: 1495–1502.
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 2005; 102: 18538–18543.
Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res 2001; 61: 5132–5136.
Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA 2007; 104: 3967–3972.
Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW . The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011; 105: 93–103.
Donia M, Ellebaek E, Andersen MH, Straten PT, Svane IM . Analysis of Vdelta1 T cells in clinical grade melanoma-infiltrating lymphocytes. Oncoimmunology 2012; 1: 1297–1304.
Donia M, Fagone P, Nicoletti F, Andersen RS, Hogdall E, Straten PT et al. BRAF inhibition improves tumor recognition by the immune system: potential implications for combinatorial therapies against melanoma involving adoptive T-cell transfer. Oncoimmunology 2012; 1: 1476–1483.
Andersen MH, Sorensen RB, Brimnes MK, Svane IM, Becker JC, thor Straten P . Identification of heme oxygenase-1-specific regulatory CD8+ T cells in cancer patients. J Clin Invest 2009; 119: 2245–2256.
Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood 2009; 113: 6102–6111.
Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE et al. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest 2006; 116: 2423–2433.
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10: 942–949.
Pollard JW . Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004; 4: 71–78.
Hussain S, He P, Subleski J, Hofseth L, Trivers G, Mechanic L et al. Nitric oxide is a key component in inflammation-accelerated tumorigenesis. Cancer Res 2008; 68: 7130–7136.
Xu W, Liu L, Loizidou M, Ahmed M, Charles I . The role of nitric oxide in cancer. Cell research 2002; 12: 311–331.
Cassina A, Radi R . Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 1996; 328: 309–316.
Brown GC, McBride AG, Fox EJ, McNaught KS, Borutaite V . Nitric oxide and oxygen metabolism. Biochem Soc Trans 1997; 25: 901–904.
Lepoivre M, Fieschi F, Coves J, Thelander L, Fontecave M . Inactivation of ribonucleotide reductase by nitric oxide. Biochem Biophys Res Commun 1991; 179: 442–448.
Nguyen T, Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR . DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci USA 1992; 89: 3030–3034.
Brito C, Naviliat M, Tiscornia AC, Vuillier F, Gualco G, Dighiero G et al. Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J Immunol 1999; 162: 3356–3366.
Radi R . Nitric oxide, oxidants and protein tyrosine nitration. Proc Natl Acad Sci USA 2004; 101: 4003–4008.
Suzuki YJ, Carini M, Butterfield DA . Protein carbonylation. Antioxid Redox Signal 2010; 12: 323–325.
Bingisser RM, Tilbrook PA, Holt PG, Kees UR . Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol 1998; 160: 5729–5734.
Xie K, Huang S, Dong Z, Juang S, Gutman M, Xie Q et al. Transfection with the inducible nitric oxide synthase gene suppresses tumorigenicity and abrogates metastasis by K-1735 murine melanoma cells. J Exp Med 1995; 181: 1333–1343.
Forrester K, Ambs S, Lupold SE, Kapust RB, Spillare EA, Weinberg WC et al. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc Natl Acad Sci USA 1996; 93: 2442–2447.
Miranda KM, Espey MG, Wink DA . A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001; 5: 62–71.
Acknowledgements
This work was supported by grants from the Ministry of Science and Technology of China (2010CB945600 and 2011DFA30630), Scientific Innovation Project of the Chinese Academy of Sciences (XDA01040107 and KSCX1-YW-22-04) and International Cooperation and Exchanges NSFC (31010103908). We are also grateful to Yikun Yao (IHS, CAS) for his assistance and suggestions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Huang, Y., Yu, P., Li, W. et al. p53 regulates mesenchymal stem cell-mediated tumor suppression in a tumor microenvironment through immune modulation. Oncogene 33, 3830–3838 (2014). https://doi.org/10.1038/onc.2013.355
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.355
Keywords
This article is cited by
-
Identification of CircRNA signature associated with tumor immune infiltration to predict therapeutic efficacy of immunotherapy
Nature Communications (2023)
-
Tp53 haploinsufficiency is involved in hotspot mutations and cytoskeletal remodeling in gefitinib-induced drug-resistant EGFRL858R-lung cancer mice
Cell Death Discovery (2023)
-
Phosphatase SHP1 impedes mesenchymal stromal cell immunosuppressive capacity modulated by JAK1/STAT3 and P38 signals
Cell & Bioscience (2020)
-
In Vivo Effects of Human Bone Marrow Mesenchymal Stromal Cells on the Development of Experimental B16 Melanoma in Mice
Bulletin of Experimental Biology and Medicine (2020)
-
Delivery of oncolytic vaccinia virus by matched allogeneic stem cells overcomes critical innate and adaptive immune barriers
Journal of Translational Medicine (2019)