Abstract
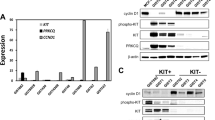
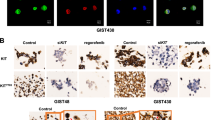
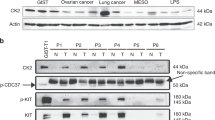
Most gastrointestinal stromal tumors (GISTs) contain KIT or PDGFRA kinase gain-of-function mutations, and therefore respond clinically to imatinib and other tyrosine kinase inhibitor (TKI) therapies. However, clinical progression subsequently results from selection of TKI-resistant clones, typically containing secondary mutations in the KIT kinase domain, which can be heterogeneous between and within GIST metastases in a given patient. TKI-resistant KIT oncoproteins require HSP90 chaperoning and are potently inactivated by HSP90 inhibitors, but clinical applications in GIST patients are constrained by the toxicity resulting from concomitant inactivation of various other HSP90 client proteins, beyond KIT and PDGFRA. To identify novel targets responsible for KIT oncoprotein function, we performed parallel genome-scale short hairpin RNA (shRNA)-mediated gene knockdowns in KIT-mutant GIST-T1 and GIST882. GIST cells were infected with a lentiviral shRNA pooled library targeting 11 194 human genes, and allowed to proliferate for 5–7 weeks, at which point assessment of relative hairpin abundance identified the HSP90 cofactor, CDC37, as one of the top six GIST-specific essential genes. Validations in treatment-naive (GIST-T1, GIST882) vs imatinib-resistant GISTs (GIST48, GIST430) demonstrated that: (1) CDC37 interacts with oncogenic KIT; (2) CDC37 regulates expression and activation of KIT and downstream signaling intermediates in GIST; and (3) unlike direct HSP90 inhibition, CDC37 knockdown accomplishes prolonged KIT inhibition (>20 days) in GIST. These studies highlight CDC37 as a key biologic vulnerability in both imatinib-sensitive and imatinib-resistant GIST. CDC37 targeting is expected to be selective for KIT/PDGFRA and a subset of other HSP90 clients, and thereby represents a promising strategy for inactivating the myriad KIT/PDGFRA oncoproteins in TKI-resistant GIST patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998; 279: 577–580.
Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003; 299: 708–710.
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002; 347: 472–480.
DeMatteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009; 373: 1097–1104.
Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004; 364: 1127–1134.
Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol 2006; 24: 4764–4774.
Antonescu CR, Besmer P, Guo T, Arkun K, Hom G, Koryotowski B et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res 2005; 11: 4182–4190.
Liegl B, Kepten I, Le C, Zhu M, Demetri GD, Heinrich MC et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol 2008; 216: 64–74.
Agaram NP, Wong GC, Guo T, Maki RG, Singer S, DeMatteo RP et al. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer 2008; 47: 853–859.
Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol 2008; 26: 5352–5359.
Antonescu CR . The GIST paradigm: lessons for other kinase-driven cancers. J Pathol 2011; 223: 251–261.
Bauer S, Yu LK, Demetri GD, Fletcher JA . Heat shock protein 90 inhibition in imatinib-resistant gastrointestinal stromal tumor. Cancer Res 2006; 66: 9153–9161.
Smyth T, Van Looy T, Curry JE, Rodriguez-Lopez AM, Wozniak A, Zhu M et al. The HSP90 inhibitor, AT13387, is effective against imatinib-sensitive and -resistant gastrointestinal stromal tumor models. Mol Cancer Ther 2012; 11: 1799–1808.
Demetri GD, Le Cesne A, Von Mehren M, Chmielowski B, Bauer S, Chow WA et al Final results from a phase III study of IPI-504 (retaspimycin hydrochloride) versus placebo in patients (pts) with gastrointestinal stromal tumors (GIST) following failure of kinase inhibitor therapies [abstract] 2010. In: Proceedings of the Gastrointestinal Cancers Symposium. American Society of Clinical Oncology, Alexandria, VA, USA, 2010.
Luo B, Cheung HW, Subramanian A, Sharifnia T, Okamoto M, Yang X et al. Highly parallel identification of essential genes in cancer cells. Proc Natl Acad Sci USA 2008; 105: 20380–20385.
Cheung HW, Cowley GS, Weir BA, Boehm JS, Rusin S, Scott JA et al. Systematic investigation of genetic vulnerabilities across cancer cell lines reveals lineage-specific dependencies in ovarian cancer. Proc Natl Acad Sci USA 2011; 108: 12372–12377.
Vaughan CK, Mollapour M, Smith JR, Truman A, Hu B, Good VM et al. Hsp90-dependent activation of protein kinases is regulated by chaperone-targeted dephosphorylation of CDC37. Mol Cell 2008; 31: 886–895.
Smith JR, Workman P . Targeting CDC37: an alternative, kinase-directed strategy for disruption of oncogenic chaperoning. Cell Cycle 2009; 8: 362–372.
Xu W, Mollapour M, Prodromou C, Wang S, Scroggins BT, Palchick Z et al. Dynamic tyrosine phosphorylation modulates cycling of the HSP90-P50(CDC37)-AHA1 chaperone machine. Mol Cell 2012; 47: 434–443.
Rubin BP, Singer S, Tsao C, Duensing A, Lux ML, Ruiz R et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res 2001; 61: 8118–8121.
Chandarlapaty S, Sawai A, Ye Q, Scott A, Silinski M, Huang K et al. SNX2112, a synthetic heat shock protein 90 inhibitor, has potent antitumor activity against HER kinase-dependent cancers. Clin Cancer Res 2008; 14: 240–248.
Zhang T, Li Y, Yu Y, Zou P, Jiang Y, Sun D . Characterization of celastrol to inhibit HSP90 and CDC37 interaction. J Biol Chem 2009; 284: 35381–35389.
Lee CH, Ou WB, Marino-Enriquez A, Zhu M, Mayeda M, Wang Y et al. 14-3-3 fusion oncogenes in high-grade endometrial stromal sarcoma. Proc Natl Acad Sci USA 2012; 17: 929–934.
Acknowledgements
Adrián Mariño-Enríquez, Wen-Bin Ou, Yuexiang Wang, Jonathan A Fletcher and George D Demetri, are supported by the GI SPORE 1P50CA12703-05, Virginia and Daniel K Ludwig Trust for Cancer Research, Paul’s Posse and Team Cesarini of the Pan Mass Challenge, LifeRaft Group and the GIST Cancer Research Fund. Adrián Mariño-Enríquez is also supported by a Sarcoma Alliance for Research Through Collaboration (SARC) Career Development Award. Wen-Bin Ou is also supported by Qianjiang Talents Project of Zhejiang (2012R10079), a grant from the Science and Technology Bureau of Jiaxing, Zhejiang (2012AY1039) and the Major Science and Technology Special Project of Zhejiang Province (2012C03007-4). The RNAi Consortium (TRC) supported the development of pooled screening methods used for these screens.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Dr George D Demetri serves as consultant for Novartis, Pfizer, Sanofi-Aventis, Glaxo-Smith-Kline, Johnson & Johnson, Merrimack Pharma, Foundation Medicine, Merck, ZioPharm, N-of-One, Champions Biotechnology, Blueprint Medicines; he is a member of the Scientific Advisory Boards of ZioPharm, Kolltan Pharmaceuticals, N-of-One, and Blueprint Medicines; and he holds minor equity stakes at N-of-One, Champions Biotechnology, Kolltan Pharmaceuticals and Blueprint Medicines. Novartis, Pfizer, Sanofi-Aventis, Glaxo-Smith-Kline, Johnson & Johnson, Merck, and Amgen support clinical trials in the Center for Sarcoma and Bone Oncology, Dana-Farber Cancer Institute. Dr Jonathan A Fletcher has consulting arrangements with Novartis, Pfizer and Deciphera Pharmaceuticals. None of these relationships constitute a conflict of interest for this work. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Mariño-Enríquez, A., Ou, WB., Cowley, G. et al. Genome-wide functional screening identifies CDC37 as a crucial HSP90-cofactor for KIT oncogenic expression in gastrointestinal stromal tumors. Oncogene 33, 1872–1876 (2014). https://doi.org/10.1038/onc.2013.127
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.127
Keywords
This article is cited by
-
Cdc37 suppression induces plasma cell immaturation and bortezomib resistance in multiple myeloma via Xbp1s
Oncogenesis (2020)
-
Coordinated targeting of CK2 and KIT in gastrointestinal stromal tumours
British Journal of Cancer (2020)
-
Novel Insights into the Treatment of Imatinib-Resistant Gastrointestinal Stromal Tumors
Targeted Oncology (2017)
-
The novel pyrrolo-1,5-benzoxazepine, PBOX-15, synergistically enhances the apoptotic efficacy of imatinib in gastrointestinal stromal tumours; suggested mechanism of action of PBOX-15
Investigational New Drugs (2016)
-
The oncogenic role of the cochaperone Sgt1
Oncogenesis (2015)