Abstract
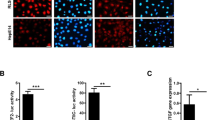
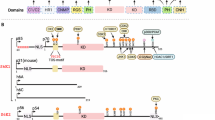
The liver kinase B1 (LKB1) tumor suppressor inhibits cell growth through its regulation of cellular metabolism and apical–basal polarity. The best understood mechanism whereby LKB1 limits cell growth is through activation of the AMP-activated-protein-kinase/mammalian-target-of-rapamycin (AMPK/mTOR) pathway to control metabolism. As LKB1 is also required for polarized epithelial cells to resist hyperplasia, it is anticipated to function through additional mechanisms. Recently, Yes-associated protein (Yap) has emerged as a transcriptional co-activator that modulates tissue homeostasis in response to cell–cell contact. Thus this study examined a possible connection between Yap and LKB1. Restoration of LKB1 expression in HeLa cells, which lack this tumor suppressor, or short-hairpin RNA knockdown of LKB1 in NTERT immortalized keratinocytes, demonstrated that LKB1 promotes Yap phosphorylation, nuclear exclusion and proteasomal degradation. The ability of phosphorylation-defective Yap mutants to rescue LKB1 phenotypes, such as reduced cell proliferation and cell size, suggest that Yap inhibition contributes to LKB1 tumor suppressor function(s). However, failure of Lats1/2 knockdown to suppress LKB1-mediated Yap regulation suggested that LKB1 signals to Yap via a non-canonical pathway. Additionally, LKB1 inhibited Yap independently of either AMPK or mTOR activation. These findings reveal a novel mechanism whereby LKB1 may restrict cancer cell growth via the inhibition of Yap.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bilder D . Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev 2004; 18: 1909–1925.
Shackelford DB, Shaw RJ . The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer 2009; 9: 563–575.
Zhao B, Li L, Lei Q, Guan KL . The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev 2010; 24: 862–874.
Hezel AF, Bardeesy N . LKB1; linking cell structure and tumor suppression. Oncogene 2008; 27: 6908–6919.
Gill RK, Yang SH, Meerzaman D, Mechanic LE, Bowman ED, Jeon HS et al. Frequent homozygous deletion of the LKB1/STK11 gene in non-small cell lung cancer. Oncogene 2011; 30: 3784–3791.
Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J 2004; 23: 833–843.
van Veelen W, Korsse SE, van de Laar L, Peppelenbosch MP . The long and winding road to rational treatment of cancer associated with LKB1/AMPK/TSC/mTORC1 signaling. Oncogene 2011; 30: 2289–2303.
Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ et al. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 2004; 116: 457–466.
Partanen JI, Nieminen AI, Makela TP, Klefstrom J . Suppression of oncogenic properties of c-Myc by LKB1-controlled epithelial organization. Proc Natl Acad Sci USA 2007; 104: 14694–14699.
Huang J, Wu S, Barrera J, Matthews K, Pan D . The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005; 122: 421–434.
Pan D . The hippo signaling pathway in development and cancer. Dev Cell 2010; 19: 491–505.
Willecke M, Hamaratoglu F, Kango-Singh M, Udan R, Chen CL, Tao C et al. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol 2006; 16: 2090–2100.
Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell 2011; 144: 782–795.
Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA 2006; 103: 12405–12410.
Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell 2010; 19: 27–38.
Bertini E, Oka T, Sudol M, Strano S, Blandino G . YAPat the crossroad between transformation and tumor suppression. Cell Cycle 2009; 8: 49–57.
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 2007; 21: 2747–2761.
Zhao B, Li L, Tumaneng K, Wang CY, Guan KL . A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev 2010; 24: 72–85.
Mauviel A, Nallet-Staub F, Varelas X . Integrating developmental signals: a Hippo in the (path)way. Oncogene 2012; 31: 1743–1756.
Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA et al. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol 2000; 20: 1436–1447.
Zhao B, Ye X, Yu J, Li L, Li W, Li S et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 2008; 22: 1962–1971.
Basu S, Totty NF, Irwin MS, Sudol M, Downward J . Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell 2003; 11: 11–23.
Bettencourt-Dias M, Giet R, Sinka R, Mazumdar A, Lock WG, Balloux F et al. Genome-wide survey of protein kinases required for cell cycle progression. Nature 2004; 432: 980–987.
Huang H, Potter CJ, Tao W, Li DM, Brogiolo W, Hafen E et al. PTEN affects cell size, cell proliferation and apoptosis during Drosophila eye development. Development 1999; 126: 5365–5372.
Granot Z, Swisa A, Magenheim J, Stolovich-Rain M, Fujimoto W, Manduchi E et al. LKB1 regulates pancreatic beta cell size, polarity and function. Cell Metab 2009; 10: 296–308.
Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 2007; 17: 2054–2060.
Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev 2010; 24: 1106–1118.
Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 2009; 284: 8023–8032.
Castro AF, Rebhun JF, Clark GJ, Quilliam LA . Rheb binds tuberous sclerosis complex 2 (TSC2) and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. J Biol Chem 2003; 278: 32493–32496.
Saucedo LJ, Edgar BA . Why size matters: altering cell size. Curr Opin Genet Dev 2002; 12: 565–571.
Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A et al. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J 2005; 24: 1810–1820.
Wada K, Itoga K, Okano T, Yonemura S, Sasaki H . Hippo pathway regulation by cell morphology and stress fibers. Development 2011; 138: 3907–3914.
Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M et al. Role of YAP/TAZ in mechanotransduction. Nature 2011; 474: 179–183.
Zong H, Kaibuchi K, Quilliam LA . The insert region of RhoA is essential for Rho kinase activation and cellular transformation. Mol Cell Biol 2001; 21: 5287–5298.
Asuri S, Yan J, Paranavitana NC, Quilliam LA . E-cadherin dis-engagement activates the Rap1 GTPase. J Cell Biochem 2008; 105: 1027–1037.
Baas AF, Smit L, Clevers H . LKB1 tumor suppressor protein: PARtaker in cell polarity. Trends Cell Biol 2004; 14: 312–319.
Humbert N, Navaratnam N, Augert A, Da Costa M, Martien S, Wang J et al. Regulation of ploidy and senescence by the AMPK-related kinase NUAK1. EMBO J 2010; 29: 376–386.
Liu L, Ulbrich J, Muller J, Wustefeld T, Aeberhard L, Kress TR et al. Deregulated MYC expression induces dependence upon AMPK-related kinase 5. Nature 2012; 483: 608–612.
Bao Y, Nakagawa K, Yang Z, Ikeda M, Withanage K, Ishigami-Yuasa M et al. A cell-based assay to screen stimulators of the Hippo pathway reveals the inhibitory effect of dobutamine on the YAP-dependent gene transcription. J Biochem 2011; 150: 199–208.
Carretero J, Medina PP, Blanco R, Smit L, Tang M, Roncador G et al. Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene 2007; 26: 1616–1625.
Hauser CA, Westwick JK, Quilliam LA . Ras-mediated transcription activation: analysis by transient cotransfection assays. Methods Enzymol 1995; 255: 412–426.
Komuro A, Nagai M, Navin NE, Sudol M . WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem 2003; 278: 33334–33341.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM . Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005; 307: 1098–1101.
Heller B, Adu-Gyamfi E, Smith-Kinnaman W, Babbey C, Vora M, Xue Y et al. Amot recognizes a juxtanuclear endocytic recycling compartment via a novel lipid binding domain. J Biol Chem 2010; 285: 12308–12320.
Ranahan WP, Han Z, Smith-Kinnaman W, Nabinger SC, Heller B, Herbert BS et al. The adaptor protein AMOT promotes the proliferation of mammary epithelial cells via the prolonged activation of the extracellular signal-regulated kinases. Cancer Res 2011; 71: 2203–2211.
Acknowledgements
We thank Dan Spandau for NTERT cells, Jacob Adler and Bill Ranahan for experimental advice. This work was supported by US Department of Defense award W81XWH1110355, the LAM Foundation and a research support funds grant from IUPUI to LAQ.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Nguyen, H., Babcock, J., Wells, C. et al. LKB1 tumor suppressor regulates AMP kinase/mTOR-independent cell growth and proliferation via the phosphorylation of Yap. Oncogene 32, 4100–4109 (2013). https://doi.org/10.1038/onc.2012.431
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.431
Keywords
This article is cited by
-
STK11 loss leads to YAP1-mediated transcriptional activation in human KRAS-driven lung adenocarcinoma cell lines
Cancer Gene Therapy (2024)
-
Non-hippo kinases: indispensable roles in YAP/TAZ signaling and implications in cancer therapy
Molecular Biology Reports (2023)
-
Hippo signalling in the liver: role in development, regeneration and disease
Nature Reviews Gastroenterology & Hepatology (2022)
-
Lkb1 deficiency confers glutamine dependency in polycystic kidney disease
Nature Communications (2018)
-
Gastrin activates autophagy and increases migration and survival of gastric adenocarcinoma cells
BMC Cancer (2017)