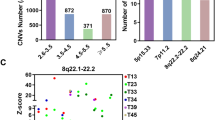
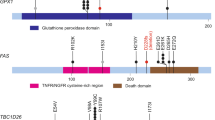
Abstract
CHEK2 encodes a serine/threonine kinase (Chk2) activated by ATM in response to DNA double-strand breaks. On the one hand, CHEK2 has been described as a tumor suppressor with proapoptotic, cell-cycle checkpoint and mitotic functions. On the other hand, Chk2 is also commonly activated (phosphorylated at T68) in cancers and precancerous lesions. Here, we report an extensive characterization of CHEK2 across the panel of 60 established cancer cell lines from the NCI Anticancer Screen (the NCI-60) using genomic and proteomic analyses, including exon-specific mRNA expression, DNA copy-number variation (CNV) by aCGH, exome sequencing, as well as western blot analyses for total and activated (pT68-Chk2) Chk2. We show that the high heterogeneity of Chk2 levels in cancer cells is primarily due to its inactivation (owing to low gene expression, alternative splicing, point mutations, copy-number alterations and premature truncation) or reduction of protein levels. Moreover, we observe that a significant percentage of cancer cells (12% of the NCI-60 and HeLa cells) show high endogenous Chk2 activation, which is always associated with p53 inactivation, and which is accompanied by downregulation of the Fanconi anemia and homologous recombination pathways. We also report the presence of activated Chk2 (pT68-Chk2) along with histone γ-H2AX in centrosomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Abdelmohsen K, Pullmann Jr R, Lal A, Kim HH, Galban S, Yang X et al. (2007). Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell 25: 543–557.
Ahn J, Prives C . (2002). Checkpoint kinase 2 (Chk2) monomers or dimers phosphorylate Cdc25C after DNA damage regardless of threonine 68 phosphorylation. J Biol Chem 277: 48418–48426.
Ahn J, Urist M, Prives C . (2004). The Chk2 protein kinase. DNA Repair (Amst) 3: 1039–1047.
Ahn JY, Li X, Davis HL, Canman CE . (2002). Phosphorylation of threonine 68 promotes oligomerization and autophosphorylation of the Chk2 protein kinase via the forkhead-associated domain. J Biol Chem 277: 19389–19395.
Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE . (2000). Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res 60: 5934–5936.
Albert JM, Cao C, Kim KW, Willey CD, Geng L, Xiao D et al. (2007). Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin Cancer Res 13: 3033–3042.
Antoni L, Sodha N, Collins I, Garrett MD . (2007). CHK2 kinase: cancer susceptibility and cancer therapy—two sides of the same coin? Nat Rev Cancer 7: 925–936.
Bahassi EM, Ovesen JL, Riesenberg AL, Bernstein WZ, Hasty PE, Stambrook PJ . (2008). The checkpoint kinases Chk1 and Chk2 regulate the functional associations between hBRCA2 and Rad51 in response to DNA damage. Oncogene 27: 3977–3985.
Bartek J, Falck J, Lukas J . (2001). CHK2 kinase—a busy messenger. Nat Rev Mol Cell Biol 2: 877–886.
Bartek J, Lukas J . (2003). Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3: 421–429.
Bartkova J, Falck J, Rajpert-De Meyts E, Skakkebaek NE, Lukas J, Bartek J . (2001). Chk2 tumour suppressor protein in human spermatogenesis and testicular germ-cell tumours. Oncogene 20: 5897–5902.
Bartkova J, Guldberg P, Gronbaek K, Koed K, Primdahl H, Moller K et al. (2004). Aberrations of the Chk2 tumour suppressor in advanced urinary bladder cancer. Oncogene 23: 8545–8551.
Bartkova J, Hamerlik P, Stockhausen MT, Ehrmann J, Hlobilkova A, Laursen H et al. (2010). Replication stress and oxidative damage contribute to aberrant constitutive activation of DNA damage signalling in human gliomas. Oncogene 29: 5095–5102.
Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K et al. (2005). DNA damage response as a candidate anticancer barrier in early human tumorigenesis. Nature 434: 864–870.
Bell DW, Varley JM, Szydlo TE, Kang DH, Wahrer DC, Shannon KE et al. (1999). Heterozygous germ line hCHK2 mutations in Li–Fraumeni syndrome. Science 286: 2528–2531.
Berge EO, Staalesen V, Straume AH, Lillehaug JR, Lonning PE . (2010). Chk2 splice variants express a dominant-negative effect on the wild-type Chk2 kinase activity. Biochim Biophys Acta 1803: 386–395.
Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S et al. (2008). GammaH2AX and cancer. Nat Rev Cancer 8: 957–967.
Bussey KJ, Chin K, Lababidi S, Reimers M, Reinhold WC, Kuo WL et al. (2006). Integrating data on DNA copy number with gene expression levels and drug sensitivities in the NCI-60 cell line panel. Mol Cancer Ther 5: 853–867.
Cao L, Kim S, Xiao C, Wang RH, Coumoul X, Wang X et al. (2006). ATM–Chk2–p53 activation prevents tumorigenesis at an expense of organ homeostasis upon Brca1 deficiency. EMBO J 25: 2167–2177.
Castedo M, Perfettini JL, Roumier T, Yakushijin K, Horne D, Medema R et al. (2004). The cell cycle checkpoint kinase Chk2 is a negative regulator of mitotic catastrophe. Oncogene 23: 4353–4361.
Chehab NH, Malikzay A, Appel M, Halazonetis TD . (2000). Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev 14: 278–288.
DiTullio Jr RA, Mochan TA, Venere M, Bartkova J, Sehested M, Bartek J et al. (2002). 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nat Cell Biol 4: 998–1002.
Eymin B, Claverie P, Salon C, Leduc C, Col E, Brambilla E et al. (2006). p14ARF activates a Tip60-dependent and p53-independent ATM/ATR/CHK pathway in response to genotoxic stress. Mol Cell Biol 26: 4339–4350.
Falck J, Mailand N, Syljuasen RG, Bartek J, Lukas J . (2001). The ATM–Chk2–Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 410: 842–847.
Fan C, Quan R, Feng X, Gillis A, He L, Matsumoto ED et al. (2006). ATM activation is accompanied with earlier stages of prostate tumorigenesis. Biochim Biophys Acta 1763: 1090–1097.
Ghosh JC, Dohi T, Raskett CM, Kowalik TF, Altieri DC . (2006). Activated checkpoint kinase 2 provides a survival signal for tumor cells. Cancer Res 66: 11576–11579.
Gire V, Roux P, Wynford-Thomas D, Brondello JM, Dulic V . (2004). DNA damage checkpoint kinase Chk2 triggers replicative senescence. EMBO J 23: 2554–2563.
Gmeiner WH, Reinhold WC, Pommier Y . (2010). Genome-wide mRNA and microRNA profiling of the NCI 60 cell-line screen and comparison of FdUMP[10] with fluorouracil, floxuridine, and topoisomerase 1 poisons. Mol Cancer Ther 9: 3105–3114.
Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T et al. (2005). Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434: 907–913.
Harper JW, Elledge SJ . (2007). The DNA damage response: ten years after. Mol Cell 28: 739–745.
Hattori H, Kuroda M, Ishida T, Shinmura K, Nagai S, Mukai K et al. (2004). Human DNA damage checkpoints and their relevance to soft tissue sarcoma. Pathol Int 54: 26–31.
Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI et al. (2004). Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res 64: 9152–9159.
Hirao A, Cheung A, Duncan G, Girard PM, Elia AJ, Wakeham A et al. (2002). Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol 22: 6521–6532.
Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H et al. (2000). DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 287: 1824–1827.
Holbeck SL, Collins JM, Doroshow JH . (2010). Analysis of food and drug administration-approved anticancer agents in the NCI60 panel of human tumor cell lines. Mol Cancer Ther 9: 1451–1460.
Ikediobi ON, Davies H, Bignell G, Edkins S, Stevens C, O'Meara S et al. (2006). Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol Cancer Ther 5: 2606–2612.
Jobson AG, Lountos GT, Lorenzi PL, Llamas J, Connelly J, Cerna D et al. (2009). Cellular inhibition of checkpoint kinase 2 (Chk2) and potentiation of camptothecins and radiation by the novel Chk2 inhibitor PV1019 [7-nitro-1H-indole-2-carboxylic acid {4-[1-(guanidinohydrazone)-ethyl]-phenyl}-amide]. J Pharmacol Exp Ther 331: 816–826.
Kessis TD, Slebos RJ, Nelson WG, Kastan MB, Plunkett BS, Han SM et al. (1993). Human papillomavirus 16 E6 expression disrupts the p53-mediated cellular response to DNA damage. Proc Natl Acad Sci USA 90: 3988–3992.
Kim DS, Kim MJ, Lee JY, Lee SM, Choi JE, Lee SY et al. (2009). Epigenetic inactivation of checkpoint kinase 2 gene in non-small cell lung cancer and its relationship with clinicopathological features. Lung Cancer 65: 247–250.
Leahy JJ, Golding BT, Griffin RJ, Hardcastle IR, Richardson C, Rigoreau L et al. (2004). Identification of a highly potent and selective DNA-dependent protein kinase (DNA-PK) inhibitor (NU7441) by screening of chromenone libraries. Bioorg Med Chem Lett 14: 6083–6087.
Lee SB, Kim SH, Bell DW, Wahrer DC, Schiripo TA, Jorczak MM et al. (2001). Destabilization of CHK2 by a missense mutation associated with Li–Fraumeni syndrome. Cancer Res 61: 8062–8067.
Li J, Stern DF . (2005). Regulation of CHK2 by DNA-dependent protein kinase. J Biol Chem 280: 12041–12050.
Li J, Taylor IA, Lloyd J, Clapperton JA, Howell S, MacMillan D et al. (2008). Chk2 oligomerization studied by phosphopeptide ligation: implications for regulation and phosphodependent interactions. J Biol Chem 283: 36019–36030.
Lukas C, Bartkova J, Latella L, Falck J, Mailand N, Schroeder T et al. (2001). DNA damage-activated kinase Chk2 is independent of proliferation or differentiation yet correlates with tissue biology. Cancer Res 61: 4990–4993.
Matsuoka S, Huang M, Elledge SJ . (1998). Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282: 1893–1897.
Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ . (2000). Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA 97: 10389–10394.
Matys V, Kel-Margoulis OV, Fricke E, Liebich I, Land S, Barre-Dirrie A et al. (2006). TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res 34: D108–D110.
Meijers-Heijboer H, van den Ouweland A, Klijn J, Wasielewski M, de Snoo A, Oldenburg R et al. (2002). Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet 31: 55–59.
Nevanlinna H, Bartek J . (2006). The CHEK2 gene and inherited breast cancer susceptibility. Oncogene 25: 5912–5919.
Ng PC, Henikoff S . (2003). SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res 31: 3812–3814.
Niida H, Murata K, Shimada M, Ogawa K, Ohta K, Suzuki K et al. (2010). Cooperative functions of Chk1 and Chk2 reduce tumour susceptibility in vivo. EMBO J 29: 3558–3570.
Nishizuka S, Charboneau L, Young L, Major S, Reinhold WC, Waltham M et al. (2003). Proteomic profiling of the NCI-60 cancer cell lines using new high-density reverse-phase lysate microarrays. Proc Natl Acad Sci USA 100: 14229–14234.
Nuciforo PG, Luise C, Capra M, Pelosi G, d'Adda di Fagagna F . (2007). Complex engagement of DNA damage response pathways in human cancer and in lung tumor progression. Carcinogenesis 28: 2082–2088.
Ou YH, Chung PH, Sun TP, Shieh SY . (2005). p53 C-terminal phosphorylation by CHK1 and CHK2 participates in the regulation of DNA-damage-induced C-terminal acetylation. Mol Biol Cell 16: 1684–1695.
Pfister TD, Reinhold WC, Agama K, Gupta S, Khin SA, Kinders RJ et al. (2009). Topoisomerase I levels in the NCI-60 cancer cell line panel determined by validated ELISA and microarray analysis and correlation with indenoisoquinoline sensitivity. Mol Cancer Ther 8: 1878–1884.
Pommier Y, Sordet O, Rao VA, Zhang H, Kohn KW . (2005). Targeting chk2 kinase: molecular interaction maps and therapeutic rationale. Curr Pharm Des 11: 2855–2872.
Pommier Y, Weinstein JN, Aladjem MI, Kohn KW . (2006). Chk2 molecular interaction map and rationale for Chk2 inhibitors. Clin Cancer Res 12: 2657–2661.
Powers JT, Hong S, Mayhew CN, Rogers PM, Knudsen ES, Johnson DG . (2004). E2F1 uses the ATM signaling pathway to induce p53 and Chk2 phosphorylation and apoptosis. Mol Cancer Res 2: 203–214.
Reinhold WC, Mergny JL, Liu H, Ryan M, Pfister TD, Kinders R et al. (2010). Exon array analyses across the NCI-60 reveal potential regulation of TOP1 by transcription pausing at guanosine quartets in the first intron. Cancer Res 70: 2191–2203.
Ribeiro-Silva A, Moutinho MA, Moura HB, Vale FR, Zucoloto S . (2006). Expression of checkpoint kinase 2 in breast carcinomas: correlation with key regulators of tumor cell proliferation, angiogenesis, and survival. Histol Histopathol 21: 373–382.
Rogoff HA, Pickering MT, Frame FM, Debatis ME, Sanchez Y, Jones S et al. (2004). Apoptosis associated with deregulated E2F activity is dependent on E2F1 and Atm/Nbs1/Chk2. Mol Cell Biol 24: 2968–2977.
Roschke AV, Tonon G, Gehlhaus KS, McTyre N, Bussey KJ, Lababidi S et al. (2003). Karyotypic complexity of the NCI-60 drug-screening panel. Cancer Res 63: 8634–8647.
Sarbia M, Ott N, Puhringer-Oppermann F, Brucher BL . (2007). The predictive value of molecular markers (p53, EGFR, ATM, CHK2) in multimodally treated squamous cell carcinoma of the oesophagus. Br J Cancer 97: 1404–1408.
Scherf U, Ross DT, Waltham M, Smith LH, Lee JK, Tanabe L et al. (2000). A gene expression database for the molecular pharmacology of cancer. Nat Genet 24: 236–244.
Schwarz JK, Lovly CM, Piwnica-Worms H . (2003). Regulation of the Chk2 protein kinase by oligomerization-mediated cis- and trans-phosphorylation. Mol Cancer Res 1: 598–609.
Sedelnikova OA, Bonner WM . (2006). GammaH2AX in cancer cells: a potential biomarker for cancer diagnostics, prediction and recurrence. Cell Cycle 5: 2909–2913.
Shankavaram UT, Reinhold WC, Nishizuka S, Major S, Morita D, Chary KK et al. (2007). Transcript and protein expression profiles of the NCI-60 cancer cell panel: an integromic microarray study. Mol Cancer Ther 6: 820–832.
Shoemaker RH . (2006). The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer 6: 813–823.
Solier S, Pommier Y . (2009). The apoptotic ring: a novel entity with phosphorylated histones H2AX and H2B and activated DNA damage response kinases. Cell Cycle 8: 1853–1859.
Solier S, Sordet O, Kohn KW, Pommier Y . (2009). Death receptor-induced activation of the Chk2- and histone H2AX-associated DNA damage response pathways. Mol Cell Biol 29: 68–82.
Staalesen V, Falck J, Geisler S, Bartkova J, Borresen-Dale AL, Lukas J et al. (2004). Alternative splicing and mutation status of CHEK2 in stage III breast cancer. Oncogene 23: 8535–8544.
Stevens C, Smith L, La Thangue NB . (2003). Chk2 activates E2F-1 in response to DNA damage. Nat Cell Biol 5: 401–409.
Stolz A, Ertych N, Kienitz A, Vogel C, Schneider V, Fritz B et al. (2010). The CHK2–BRCA1 tumour suppressor pathway ensures chromosomal stability in human somatic cells. Nat Cell Biol 12: 492–499.
Sullivan A, Yuille M, Repellin C, Reddy A, Reelfs O, Bell A et al. (2002). Concomitant inactivation of p53 and Chk2 in breast cancer. Oncogene 21: 1316–1324.
Sun Y, Jiang X, Chen S, Fernandes N, Price BD . (2005). A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci USA 102: 13182–13187.
Takai H, Naka K, Okada Y, Watanabe M, Harada N, Saito S et al. (2002). Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J 21: 5195–5205.
Tsvetkov L, Xu X, Li J, Stern DF . (2003). Polo-like kinase 1 and Chk2 interact and co-localize to centrosomes and the midbody. J Biol Chem 278: 8468–8475.
Yang S, Jeong JH, Brown AL, Lee CH, Pandolfi PP, Chung JH et al. (2006). Promyelocytic leukemia activates Chk2 by mediating Chk2 autophosphorylation. J Biol Chem 281: 26645–26654.
Yee C, Krishnan-Hewlett I, Baker CC, Schlegel R, Howley PM . (1985). Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am J Pathol 119: 361–366.
Yu Q, Rose JH, Zhang H, Pommier Y . (2001). Antisense inhibition of Chk2/hCds1 expression attenuates DNA damage-induced S and G2 checkpoints and enhances apoptotic activity in HEK-293 cells. FEBS Lett 505: 7–12.
Zhang S, Hemmerich P, Grosse F . (2007). Centrosomal localization of DNA damage checkpoint proteins. J Cell Biochem 101: 451–465.
Zhao H, Tanaka T, Halicka HD, Traganos F, Zarebski M, Dobrucki J et al. (2007). Cytometric assessment of DNA damage by exogenous and endogenous oxidants reports aging-related processes. Cytometry A 71: 905–914.
Zhao H, Traganos F, Darzynkiewicz Z . (2008). Kinetics of histone H2AX phosphorylation and Chk2 activation in A549 cells treated with topotecan and mitoxantrone in relation to the cell cycle phase. Cytometry A 73: 480–489.
Zhou M, Meng Z, Jobson AG, Pommier Y, Veenstra TD . (2007). Detection of in vitro kinase generated protein phosphorylation sites using gamma[18O4]-ATP and mass spectrometry. Anal Chem 79: 7603–7610.
Acknowledgements
The present data were in part presented in abstract form at the 21st EORTC-NCI-AACR International Symposium on Molecular Targets and Cancer Therapeutics (Boston, 15–19 November 2009) and at the 101st AACR Annual Meeting (Washington DC, 17–21 April 2010). We thank Sven Bilke, Yuan Jiang, Marbin Pineda, Robert Walker and Yuelin Zhu for technical help in next-generation sequencing and data analysis, and Jaleisa Turner for help in capillary sequencing. We also thank Margot Sunshine (LMP BioInformatics Groups) for invaluable work and technical help. GZ thanks Dr P Blandini for insightful suggestions on the possible consequences of Chk2 activation in cancer treatment. Funding: This work was supported by the Center for Cancer Research, Intramural Program of the National Cancer Institute, and by the Division of Cancer Treatment and Diagnosis, Extramural Program of the National Cancer Institute (National Institutes of Health), and by AIRC MFAG grant no. 10570 (GZ). GZ was also supported by a PhD fellowship grant (XXIII Ciclo) from the University of Genova (Genova, Italy).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Zoppoli, G., Solier, S., Reinhold, W. et al. CHEK2 genomic and proteomic analyses reveal genetic inactivation or endogenous activation across the 60 cell lines of the US National Cancer Institute. Oncogene 31, 403–418 (2012). https://doi.org/10.1038/onc.2011.283
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.283