Abstract
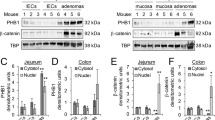
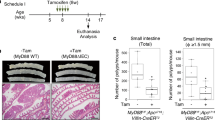
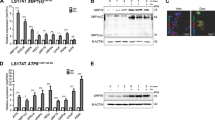
Mutation of the tumor suppressor adenomatous polyposis coli (APC) is considered an initiating step in the genesis of the vast majority of colorectal cancers. APC inhibits the Wnt-signaling pathway by targeting the proto-oncogene β-catenin for destruction by cytoplasmic proteasomes. In the presence of a Wnt signal, or in the absence of functional APC, β-catenin can serve as a transcription cofactor for genes required for cell proliferation such as cyclin-D1 and c-Myc. In cultured cells, APC shuttles between the nucleus and the cytoplasm, with nuclear APC implicated in the inhibition of Wnt target gene expression. Adopting a genetic approach to evaluate the functions of nuclear APC in the context of a whole organism, we generated a mouse model with mutations that inactivate the nuclear localization signals (NLSs) of Apc (ApcmNLS). ApcmNLS/mNLS mice are viable and fractionation of mouse embryonic fibroblasts (MEFs) isolated from these mice revealed a significant reduction in nuclear Apc as compared with Apc+/+ MEFs. The levels of Apc and β-catenin protein were not significantly altered in small intestinal epithelia from ApcmNLS/mNLS mice. Compared with Apc+/+ mice, ApcmNLS/mNLS mice showed increased proliferation in epithelial cells from the jejunum, ileum and colon. These same tissues from ApcmNLS/mNLS mice showed more mRNA from three genes upregulated in response to canonical Wnt signal, c-Myc, axin-2 and cyclin-D1, and less mRNA from Hath-1, which is downregulated in response to Wnt. These observations suggest a role for nuclear Apc in the inhibition of canonical Wnt signaling and the control of epithelial proliferation in intestinal tissue. Furthermore, we found ApcMin/+ mice, which harbor a mutation that truncates Apc, to have an increased polyp size and multiplicity if they also carry the ApcmNLS allele. Taken together, this analysis of the novel ApcmNLS mouse model supports a role for nuclear Apc in the control of Wnt target genes, intestinal epithelial cell proliferation and polyp formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Anderson CB, Neufeld KL, White RL . (2002). Subcellular distribution of Wnt pathway proteins in normal and neoplastic colon. Proc Natl Acad Sci USA 99: 8683–8688.
Bunting M, Bernstein KE, Greer JM, Capecchi MR, Thomas KR . (1999). Targeting genes for self-excision in the germ line. Genes Dev 13: 1524–1528.
Cheung AF, Carter AM, Kostova KK, Woodruff JF, Crowley D, Bronson RT et al. (2009). Complete deletion of Apc results in severe polyposis in mice. Oncogene 29: 1857–1864.
Colnot S, Niwa-Kawakita M, Hamard G, Godard C, Le Plenier S, Houbron C et al. (2004). Colorectal cancers in a new mouse model of familial adenomatous polyposis: influence of genetic and environmental modifiers. Lab Invest 84: 1619–1630.
Dingwall C, Sharnick SV, Laskey RA . (1982). A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell 30: 449–458.
Fagman H, Larsson F, Arvidsson Y, Meuller J, Nordling M, Martinsson T et al. (2003). Nuclear accumulation of full-length and truncated adenomatous polyposis coli protein in tumor cells depends on proliferation. Oncogene 22: 6013–6022.
Fodde R, Edelmann W, Yang K, van Leeuwen C, Carlson C, Renault B et al. (1994). A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci USA 91: 8969–8973.
Fodde R, Kuipers J, Rosenberg C, Smits R, Kielman M, Gaspar C et al. (2001). Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol 3: 433–438.
Galea MA, Eleftheriou A, Henderson BR . (2001). ARM domain-dependent nuclear import of adenomatous polyposis coli protein is stimulated by the B56 alpha subunit of protein phosphatase 2A. J Biol Chem 276: 45833–45839.
Giles RH, van Es JH, Clevers H . (2003). Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 1653: 1–24.
Gorlich D, Prehn S, Laskey RA, Hartmann E . (1994). Isolation of a protein that is essential for the first step of nuclear protein import. Cell 79: 767–778.
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT et al. (1998). Identification of c-MYC as a target of the APC pathway. Science 281: 1509–1512.
Henderson B . (2000). Nuclear–cytoplasmic shuttling of APC regulates β-catenin subcellular localization and turnover. Nat Cell Biol 2: 653–660.
Ishikawa T-O, Tamai Y, Li Q, Oshima M, Taketo MM . (2003). Requirement for tumor suppressor Apc in the morphogenesis of anterior and ventral mouse embryo. Dev Biol 253: 230–246.
Jaiswal AS, Narayan S . (2008). A novel function of adenomatous polyposis coli (APC) in regulating DNA repair. Cancer Lett 271: 272–280.
Kan Y, Winfried E, Kunhua F, Kirkland L, Venkateswara RK, Riccardo F et al. (1997). A mouse model of human familial adenomatous polyposis. J Exp Zool 277: 245–254.
Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Nathke IS . (2001). A role for the adenomatous polyposis coli protein in chromosome segregation. Nat Cell Biol 3: 429–432.
Kielman MF, Rindapaa M, Gaspar C, van Poppel N, Breukel C, van Leeuwen S et al. (2002). Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. Nat Genet 32: 594–605.
Magnus HA . (1937). Observations on the presence of intestinal epithelium in the gastric mucosa. J Pathol Baceriol 44: 389–398.
Mattaj IW, Englmeier L . (1998). Nucleocytoplasmic transport: the soluble phase. Annu Rev Biochem 67: 265–306.
Miyoshi Y, Ando H, Nagase H, Nishisho I, Horii A, Miki Y et al. (1992a). Germ-line mutations of the APC gene in 53 familial adenomatous polyposis patients. Proc Natl Acad Sci USA 89: 4452–4456.
Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S et al. (1992b). Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet 1: 229–233.
Moser A, Pitot H, Dove W . (1990). A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247: 322–324.
Moser AR, Mattes EM, Dove WF, Lindstrom MJ, Haag JD, Gould MN . (1993). ApcMin, a mutation in the murine Apc gene, predisposes to mammary carcinomas and focal alveolar hyperplasias. Proc Natl Acad Sci USA 90: 8977–8981.
Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P . (1995). Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA 92: 3046–3050.
Nagy A, Gertsenstein M, Vintersten K, Behringer R . (2003). Manipulating the Mouse Embryo: a Laboratory Manual 3rd edn. Cold Spring Harbor Press: New York.
Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC . (1993). Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA 90: 8424–8428.
Nathke IS, Adams CL, Polakis P, Sellin JH, Nelson J . (1996). The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J Cell Biol 134: 165–179.
Neufeld KL . (2009). Nuclear functions of APC. Adv Exp Med Biol 656: 13–29.
Neufeld KL, White RL . (1997). Nuclear and cytoplasmic localizations of the adenomatous polyposis coli protein. Proc Natl Acad Sci USA 94: 3034–3039.
Neufeld KL, Zhang F, Cullen BR, White RL . (2000). APC-mediated downregulation of β-catenin activity involves nuclear sequestration, nuclear export. EMBO Rep 1: 519–523.
Neufeld KL, Zhang F, Cullen BR, White RL . (2000). APC-mediated downregulation of beta-catenin activity involves nuclear sequestration, nuclear export. EMBO Rep 1: 519–523.
Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M . (1995). Loss of Apc heterozygosity, abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc Natl Acad Sci USA 92: 4482–4486.
Peters R . (1986). Fluorescence microphotolysis to measure nucleocytoplasmic transport, intracellular mobility. Biochim Biophys Acta 864: 305–359.
Pollard P, Deheragoda M, Segditsas S, Lewis A, Rowan A, Howarth K et al. (2009). The Apc1322 T mouse develops severe polyposis associated with submaximal nuclear β-catenin expression. Gastroenterology 136: 2204–2213.
Qian J, Sarnaik AA, Bonney TM, Keirsey J, Combs KA, Steigerwald K et al. (2008). The APC tumor suppressor inhibits DNA replication by directly binding to DNA via its carboxyl terminus. Gastroenterology 135: 152–162.
Rosin-Arbesfeld R, Cliffe A, Brabletz T, Bienz M . (2003). Nuclear export of the APC tumour suppressor controls beta-catenin function in transcription. EMBO J 22: 1101–1113.
Rosin-Arbfeld R, Townsley F, Bienz M . (2000). The APC tumour suppressor has a nuclear export function. Nature 406: 1009–1012.
Salic A, Mitchison TJ . (2008). A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA 105: 2415–2420.
Sasai H, Masaki M, Wakitani K . (2000). Suppression of polypogenesis in a new mouse strain with a truncated Apc(Delta474) by a novel COX-2 inhibitor, JTE-522. Carcinogenesis 21: 953–958.
Sena P, Saviano M, Monni S, Losi L, Roncucci L, Marzona L et al. (2006). Subcellular localization of beta-catenin and APC proteins in colorectal preneoplastic and neoplastic lesions. Cancer Lett 241: 203–212.
Sierra J, Yoshida T, Joazeiro CA, Jones KA . (2006). The APC tumor suppressor counteracts beta-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev 20: 586–600.
Smith KJ, Johnson KA, Bryan TM, Hill DE, Markowitz S, Willson JK et al. (1993). The APC gene product in normal and tumor cells. Proc Natl Acad Sci USA 90: 2846–2850.
Smits R, Kielman MF, Breukel C, Zurcher C, Neufeld K, Jagmohan-Changur S et al. (1999). Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev 13: 1309–1321.
Taketo MM . (2006). Mouse models of gastrointestinal tumors. Cancer Sci 97: 355–361.
Tetsu O, McCormick F . (1999). Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398: 422–426.
Wang Y, Azuma Y, Friedman DB, Coffey R, Neufeld KL . (2009). Novel association of APC with intermediate filaments identified using a new versatile APC antibody. BMC Cell Biol 10: 75–88.
Wang Y, Azuma Y, Moore D, Osheroff N, Neufeld KL . (2008). Interaction between tumor suppressor APC and topoisomerase IIα: implication for the G2/M transition. Mol Biol Cell 19: 4076–4085.
Whitehead RH, Demmler K, Rockman SP, Watson NK . (1999). Clonogenic growth of epithelial cells from normal colonic mucosa from both mice and humans. Gastroenterology 117: 858–865.
Zhang F, White R, Neufeld K . (2000). Phosphorylation near nuclear localization signal regulates nuclear import of APC protein. Proc Natl Acad Sci USA 97: 12577–12582.
Zhang F, White RL, Neufeld KL . (2001). Cell density and phosphorylation control the subcellular localization of adenomatous polyposis coli protein. Mol Cell Biol 21: 8143–8156.
Acknowledgements
This work was supported by RO1 CA10922 from the National Cancer Institute and 5P20 RR15563. We extend our gratitude to Alan Godwin, Kirk Thomas and Mario Capecchi for advice on the generation of the knock-in mouse and for the lambda phage library, and the tACE-Cre-Neor- and TkHSV-containing constructs. We thank Marc Roth, Areli Monarrez, Ashrita Abraham and Travis Friesen for technical assistance; the staff at the University of Kansas Animal Care Unit for excellent mouse husbandry and David Davido for critical reading of the manuscript. We also thank Reka Nagy and Drs Andras Nagy, Janet Rossant and Wanda Abramow-Newerly (Mount Sinai Hospital) for the mouse R1 ES cells.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Zeineldin, M., Cunningham, J., McGuinness, W. et al. A knock-in mouse model reveals roles for nuclear Apc in cell proliferation, Wnt signal inhibition and tumor suppression. Oncogene 31, 2423–2437 (2012). https://doi.org/10.1038/onc.2011.434
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.434
Keywords
This article is cited by
-
Comprehensive analysis of β-catenin target genes in colorectal carcinoma cell lines with deregulated Wnt/β-catenin signaling
BMC Genomics (2014)
-
Animal models of colorectal cancer
Cancer and Metastasis Reviews (2013)