Abstract
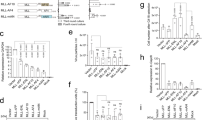
The paired box domain of PAX5 was reported to fuse with the sequence of promyelocytic leukemia (PML) to produce PAX5–PML chimeric protein in two patients with B-cell acute lymphoblastic leukemia. In the present studies, we found, by gel shift assays, that PAX5–PML bound to a panel of PAX5-consensus sequence acts as a homodimer with reduction of its DNA-binding affinities in comparison with wild-type PAX5. In transient transfection assays using 293T and HeLa cells, and retrovirus transduction of murine hematopoietic stem/progenitor cells together with quantitative real-time polymerase chain reaction analysis, PAX5–PML inhibited wild-type PAX5 target gene transcriptional activity. Studies comparing PAX5–PML with PAX5–PML(ΔCC) demonstrated that the coiled-coil (CC) protein interaction domain located within the PML moiety was required for PAX5–PML homodimer complex formation and partial transcriptional repression of genes controlled by PAX5. Fluorescent microscopic examination of transiently expressed YFP-tagged proteins in HeLa and 293T cells demonstrated that YFP–PAX5–PML and YFP–PAX5–PML(ΔCC) exhibited a diffuse granular pattern within the nucleus, similar to PAX5 but not PML. By fluorescent recovery after photobleach (FRAP), we have shown that PAX5–PML fusion protein has reduced intranuclear mobility compared with wild-type PAX5. Furthermore, the dimerization domain (CC) of PML was responsible for the reduced intranuclear mobility of PAX5–PML. These results indicate that the CC domain of PAX5–PML is important for each of the known activities of PAX5–PML fusion proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Adams B, Dorfler P, Aguzzi A, Kozmik Z, Urbanek P, Maurer-Fogy I et al. (1992). Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev 6: 1589–1607.
Barr FG . (2001). Gene fusions involving PAX and FOX family members in alveolar rhabdomyosarcoma. Oncogene 20: 5736–5746.
Cobaleda C, Schebesta A, Delogu A, Busslinger M . (2007). Pax5: the guardian of B cell identity and function. Nat Immunol 8: 463–470.
Czerny T, Schaffner G, Busslinger M . (1993). DNA sequence recognition by Pax proteins: bipartite structure of the paired domain and its binding site. Genes Dev 7: 2048–2061.
de The H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A . (1991). The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell 66: 675–684.
Delogu A, Schebesta A, Sun Q, Aschenbrenner K, Perlot T, Busslinger M . (2006). Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity 24: 269–281.
Dong S, Qiu J, Stenoien DL, Brinkley WR, Mancini MA, Tweardy DJ . (2003). Essential role for the dimerization domain of NuMA-RARalpha in its oncogenic activities and localization to NuMA sites within the nucleus. Oncogene 22: 858–868.
Dong S, Stenoien DL, Qiu J, Mancini MA, Tweardy DJ . (2004). Reduced intranuclear mobility of APL fusion proteins accompanies their mislocalization and results in sequestration and decreased mobility of retinoid X receptor alpha. Mol Cell Biol 24: 4465–4475.
Grignani F, Gelmetti V, Fanelli M, Rogaia D, De Matteis S, Ferrara FF et al. (1999). Formation of PML /RAR alpha high molecular weight nuclear complexes through the PML coiled-coil region is essential for the PML /RAR alpha-mediated retinoic acid response. Oncogene 18: 6313–6321.
Huang Y, Qiu J, Chen G, Dong S . (2008). Coiled-coil domain of PML is essential for the aberrant dynamics of PML-RARalpha, resulting in sequestration and decreased mobility of SMRT. Biochem Biophys Res Commun 365: 258–265.
Kawamata N, Ogawa S, Zimmermann M, Niebuhr B, Stocking C, Sanada M et al. (2008). Cloning of genes involved in chromosomal translocations by high-resolution single nucleotide polymorphism genomic microarray. Proc Natl Acad Sci USA 105: 11921–11926.
Koken MH, Puvion-Dutilleul F, Guillemin MC, Viron A, Linares-Cruz G, Stuurman N et al. (1994). The t(15;17) translocation alters a nuclear body in a retinoic acid-reversible fashion. EMBO J 13: 1073–1083.
Kwok C, Zeisig BB, Qiu J, Dong S, So CW . (2009). Transforming activity of AML1-ETO is independent of CBFbeta and ETO interaction but requires formation of homo-oligomeric complexes. Proc Natl Acad Sci USA 106: 2853–2858.
Lauring J, Schlissel MS . (1999). Distinct factors regulate the murine RAG-2 promoter in B- and T-cell lines. Mol Cell Biol 19: 2601–2612.
Look AT . (1997). Oncogenic transcription factors in the human acute leukemias. Science 278: 1059–1064.
Melnick A, Licht JD . (1999). Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood 93: 3167–3215.
Mikkola I, Heavey B, Horcher M, Busslinger M . (2002). Reversion of B cell commitment upon loss of Pax5 expression. Science 297: 110–113.
Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD et al. (2007). Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 446: 758–764.
Nebral K, Denk D, Attarbaschi A, Konig M, Mann G, Haas OA et al. (2009). Incidence and diversity of PAX5 fusion genes in childhood acute lymphoblastic leukemia. Leukemia 23: 134–143.
Nebral K, Konig M, Harder L, Siebert R, Haas OA, Strehl S . (2007). Identification of PML as novel PAX5 fusion partner in childhood acute lymphoblastic leukaemia. Br J Haematol 139: 269–274.
Nutt SL, Heavey B, Rolink AG, Busslinger M . (1999). Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature 401: 556–562.
Pridans C, Holmes ML, Polli M, Wettenhall JM, Dakic A, Corcoran LM et al. (2008). Identification of Pax5 target genes in early B cell differentiation. J Immunol 180: 1719–1728.
Qiu J, Gunaratne P, Peterson LE, Khurana D, Walsham N, Loulseged H et al. (2003). Novel potential ALL low-risk markers revealed by gene expression profiling with new high-throughput SSH-CCS-PCR. Leukemia 17: 1891–1900.
Qiu J, Huang Y, Chen G, Chen Z, Tweardy DJ, Dong S . (2007). Aberrant chromatin remodeling by retinoic acid receptor alpha fusion proteins assessed at the single-cell level. Mol Biol Cell 18: 3941–3951.
Qiu J, Shi G, Jia Y, Li J, Wu M, Dong S et al. (2010a). The X-linked mental retardation gene PHF8 is a histone demethylase involved in neuronal differentiation. Cell Res 20: 908–918.
Qiu J, Wong J, Tweardy DJ, Dong S . (2006). Decreased intranuclear mobility of acute myeloid leukemia 1-containing fusion proteins is accompanied by reduced mobility and compartmentalization of core binding factor beta. Oncogene 25: 3982–3993.
Qiu JJ, Lu X, Zeisig BB, Ma Z, Cai X, Chen S et al. (2010b). Leukemic transformation by the APL fusion protein PRKAR1A-RARalpha critically depends on recruitment of RXRalpha. Blood 115: 643–652.
Robson EJ, He SJ, Eccles MR . (2006). A PANorama of PAX genes in cancer and development. Nat Rev Cancer 6: 52–62.
Schebesta A, McManus S, Salvagiotto G, Delogu A, Busslinger GA, Busslinger M . (2007). Transcription factor Pax5 activates the chromatin of key genes involved in B cell signaling, adhesion, migration, and immune function. Immunity 27: 49–63.
Schebesta M, Pfeffer PL, Busslinger M . (2002). Control of pre-BCR signaling by Pax5-dependent activation of the BLNK gene. Immunity 17: 473–485.
So CW, Cleary ML . (2004). Dimerization: a versatile switch for oncogenesis. Blood 104: 919–922.
So CW, Karsunky H, Passegue E, Cozzio A, Weissman IL, Cleary ML . (2003a). MLL-GAS7 transforms multipotent hematopoietic progenitors and induces mixed lineage leukemias in mice. Cancer Cell 3: 161–171.
So CW, Lin M, Ayton PM, Chen EH, Cleary ML . (2003b). Dimerization contributes to oncogenic activation of MLL chimeras in acute leukemias. Cancer Cell 4: 99–110.
Urbanek P, Wang ZQ, Fetka I, Wagner EF, Busslinger M . (1994). Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5 /BSAP. Cell 79: 901–912.
Zeisig BB, Kwok C, Zelent A, Shankaranarayanan P, Gronemeyer H, Dong S et al. (2007). Recruitment of RXR by Homotetrameric RARalpha fusion proteins is essential for transformation. Cancer Cell 12: 36–51.
Acknowledgements
We thank Dr M Busslinger (Research Institute of Molecular Pathology, Vienna, Austria) for the CD19-luciferase and PAX5 expression vectors, Dr AS Belmont (University of Illinois Champagne-Urbana) for the A03_1 cell line, and Dr CWE So (King's College London, London, UK) for critical reading of the manuscript. This work was supported in part, by funds from the Albert and Margaret alkek Foundation and Leukemia Research Foundation (LRF).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Qiu, J., Chu, H., Lu, X. et al. The reduced and altered activities of PAX5 are linked to the protein–protein interaction motif (coiled-coil domain) of the PAX5–PML fusion protein in t(9;15)-associated acute lymphocytic leukemia. Oncogene 30, 967–977 (2011). https://doi.org/10.1038/onc.2010.473
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.473
Keywords
This article is cited by
-
FBN30 in wild Anopheles gambiae functions as a pathogen recognition molecule against clinically circulating Plasmodium falciparum in malaria endemic areas in Kenya
Scientific Reports (2017)
-
Critical role of retinoid/rexinoid signaling in mediating transformation and therapeutic response of NUP98-RARG leukemia
Leukemia (2015)
-
Dominant-negative mechanism of leukemogenic PAX5 fusions
Oncogene (2012)