Abstract
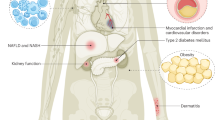
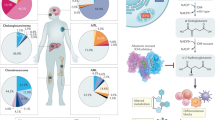
The liver kinase B1 (LKB1)/adenosine mono-phosphate-activated protein kinase (AMPK)/tuberous sclerosis complex (TSC)/mammalian target of rapamycin (mTOR) complex (mTORC1) cassette constitutes a canonical signaling pathway that integrates information on the metabolic and nutrient status and translates this into regulation of cell growth. Alterations in this pathway are associated with a wide variety of cancers and hereditary hamartoma syndromes, diseases in which hyperactivation of mTORC1 has been described. Specific mTORC1 inhibitors have been developed for clinical use, and these drugs have been anticipated to provide efficient treatment for these diseases. In the present review, we provide an overview of the metabolic LKB1/AMPK/TSC/mTORC1 pathway, describe how its aberrant signaling associates with cancer development, and indicate the difficulties encountered when biochemical data are extrapolated to provide avenues for rational treatment of disease when targeting this signaling pathway. A careful examination of preclinical and clinical studies performed with rapamycin or derivatives thereof shows that although results are encouraging, we are only half way in the long and winding road to design rationale treatment targeted at the LKB1/AMPK/TSC/mTORC1 pathway. Inherited cancer syndromes associated with this pathway such as the Peutz–Jeghers syndrome and TSC, provide perfect models to study the relationship between genetics and disease phenotype, and to delineate the complexities that underlie translation of biochemical and genetical information to clinical management, and thus provide important clues for devising novel rational medicine for cancerous diseases in general.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Abbreviations
- AML:
-
angiomyolipoma
- AMPK:
-
adenosine mono-phosphate-activated protein kinase
- FDA:
-
Food and Drug Administration
- LAM:
-
lymphangioleiomyomatosis
- LKB1:
-
liver kinase B1
- mTOR:
-
mammalian target of rapamycin
- mTORC1:
-
mTOR complex 1
- NET:
-
neuroendocrine tumor
- NF1:
-
neurofibromatosis type 1
- PJS:
-
Peutz-Jeghers syndrome
- RCC:
-
renal cell carcinoma
- SEGA:
-
subependymal giant cell astrocytoma
- TSC:
-
tuberous sclerosis complex
References
Alessi DR, Sakamoto K, Bayascas JR . (2006). LKB1-dependent signaling pathways. Annu Rev Biochem 75: 137–163.
Altomare DA, Testa JR . (2005). Perturbations of the AKT signaling pathway in human cancer. Oncogene 24: 7455–7464.
Baas AF, Boudeau J, Sapkota GP, Smit L, Medema R, Morrice NA et al. (2003). Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J 22: 3062–3072.
Baybis M, Yu J, Lee A, Golden JA, Weiner H, McKhann II G et al. (2004). mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann Neurol 56: 478–487.
Benvenuto G, Li S, Brown SJ, Braverman R, Vass WC, Cheadle JP et al. (2000). The tuberous sclerosis-1 (TSC1) gene product hamartin suppresses cell growth and augments the expression of the TSC2 product tuberin by inhibiting its ubiquitination. Oncogene 19: 6306–6316.
Bhaskar PT, Hay N . (2007). The two TORCs and Akt. Dev Cell 12: 487–502.
Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM et al. (2008). Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 358: 140–151.
Boudeau J, Baas AF, Deak M, Morrice NA, Kieloch A, Schutkowski M et al. (2003). MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J 22: 5102–5114.
Brajenovic M, Joberty G, Kuster B, Bouwmeester T, Drewes G . (2004). Comprehensive proteomic analysis of human par protein complexes reveals an interconnected protein network. J Biol Chem 279: 12804–12811.
Calva D, Howe JR . (2008). Hamartomatous polyposis syndromes. Surg Clin North Am 88: 779–817, vii.
Carling D, Zammit VA, Hardie DG . (1987). A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett 223: 217–222.
Carretero J, Medina PP, Blanco R, Smit L, Tang M, Roncador G et al. (2007). Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene 26: 1616–1625.
Carsillo T, Astrinidis A, Henske EP . (2000). Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA 97: 6085–6090.
Chan HY, Grossman AB, Bukowski RM . (2010). Everolimus in the treatment of renal cell carcinoma and neuroendocrine tumors. Adv Ther 27: 495–511.
Chan JA, Zhang H, Roberts PS, Jozwiak S, Wieslawa G, Lewin-Kowalik J et al. (2004). Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J Neuropathol Exp Neurol 63: 1236–1242.
Chong-Kopera H, Inoki K, Li Y, Zhu T, Garcia-Gonzalo FR, Rosa JL et al. (2006). TSC1 stabilizes TSC2 by inhibiting the interaction between TSC2 and the HERC1 ubiquitin ligase. J Biol Chem 281: 8313–8316.
Chung J, Kuo CJ, Crabtree GR, Blenis J . (1992). Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell 69: 1227–1236.
Consortium ECTS . (1993). Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 75: 1305–1315.
Contreras CM, Gurumurthy S, Haynie JM, Shirley LJ, Akbay EA, Wingo SN et al. (2008). Loss of Lkb1 provokes highly invasive endometrial adenocarcinomas. Cancer Res 68: 759–766.
Cook JD, Walker CL . (2004). The Eker rat: establishing a genetic paradigm linking renal cell carcinoma and uterine leiomyoma. Curr Mol Med 4: 813–824.
Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL . (2004). Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev 18: 1533–1538.
Corsenca A, Aebersold F, Moch H, Bird P, Weber M, Hofbauer G et al. (2007). Combined nephrectomy and pre-emptive renal transplantation in a tuberous sclerosis patient with angiomyolipoma, renal carcinoma and life-threatening abdominal haemorrhages. Nephrol Dial Transplant 22: 3330–3333.
Curatolo P, Bombardieri R, Jozwiak S . (2008). Tuberous sclerosis. Lancet 372: 657–668.
Davies DM, Johnson SR, Tattersfield AE, Kingswood JC, Cox JA, McCartney DL et al. (2008). Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med 358: 200–203.
Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G . (2001). Mammalian TOR: a homeostatic ATP sensor. Science 294: 1102–1105.
Dorfman J, Macara IG . (2008). STRADalpha regulates LKB1 localization by blocking access to importin-alpha, and by association with Crm1 and exportin-7. Mol Biol Cell 19: 1614–1626.
Dumont FJ, Melino MR, Staruch MJ, Koprak SL, Fischer PA, Sigal NH . (1990). The immunosuppressive macrolides FK-506 and rapamycin act as reciprocal antagonists in murine T cells. J Immunol 144: 1418–1424.
Dworakowska D, Grossman AB . (2009). Are neuroendocrine tumours a feature of tuberous sclerosis? A systematic review. Endocr Relat Cancer 16: 45–58.
Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ et al. (2008). Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med 14: 843–848.
El-Hashemite N, Walker V, Zhang H, Kwiatkowski DJ . (2003). Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin. Cancer Res 63: 5173–5177.
Ess KC . (2010). Tuberous sclerosis complex: a brave new world? Curr Opin Neurol 23: 189–193.
Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethuraman G et al. (2006). Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol 59: 490–498.
Godlewski J, Nowicki MO, Bronisz A, Nuovo G, Palatini J, De Lay M et al. (2010). MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell 37: 620–632.
Gurumurthy S, Hezel AF, Sahin E, Berger JH, Bosenberg MW, Bardeesy N . (2008). LKB1 deficiency sensitizes mice to carcinogen-induced tumorigenesis. Cancer Res 68: 55–63.
Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS et al. (2008). AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226.
Habib SL, Bhandari B, Sadek N, Abboudwerner S, Abboud HE . (2010). Novel mechanism of regulation of the DNA repair enzyme OGG1 in tuberin-deficient Cells. Carcinogenesis 31: 2022–2030.
Haemel AK, O'Brian AL, Teng JM . (2010). Topical rapamycin: a novel approach to facial angiofibromas in tuberous sclerosis. Arch Dermatol 146: 715–718.
Haidinger M, Hecking M, Weichhart T, Poglitsch M, Enkner W, Vonbank K et al. (2010). Sirolimus in renal transplant recipients with tuberous sclerosis complex: clinical effectiveness and implications for innate immunity. Transpl Int 23: 777–785.
Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S et al. (2002). Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177–189.
Hardie DG . (2007). AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8: 774–785.
Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP et al. (2003). Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2: 28.
Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG . (1995). 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem 270: 27186–27191.
Hay N, Sonenberg N . (2004). Upstream and downstream of mTOR. Genes Dev 18: 1926–1945.
Hegedus B, Banerjee D, Yeh TH, Rothermich S, Perry A, Rubin JB et al. (2008). Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res 68: 1520–1528.
Heitman J, Movva NR, Hall MN . (1991). Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253: 905–909.
Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A et al. (1998). A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 391: 184–187.
Herry I, Neukirch C, Debray MP, Mignon F, Crestani B . (2007). Dramatic effect of sirolimus on renal angiomyolipomas in a patient with tuberous sclerosis complex. Eur J Intern Med 18: 76–77.
Hezel AF, Bardeesy N . (2008). LKB1; linking cell structure and tumor suppression. Oncogene 27: 6908–6919.
Hezel AF, Gurumurthy S, Granot Z, Swisa A, Chu GC, Bailey G et al. (2008). Pancreatic LKB1 deletion leads to acinar polarity defects and cystic neoplasms. Mol Cell Biol 28: 2414–2425.
Hino O . (2004). Multistep renal carcinogenesis in the Eker (Tsc 2 gene mutant) rat model. Curr Mol Med 4: 807–811.
Hofbauer GF, Marcollo-Pini A, Corsenca A, Kistler AD, French LE, Wuthrich RP et al. (2008). The mTOR inhibitor rapamycin significantly improves facial angiofibroma lesions in a patient with tuberous sclerosis. Br J Dermatol 159: 473–475.
Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y et al. (2009). Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 20: 1981–1991.
Huang J, Manning BD . (2008). The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J 412: 179–190.
Huang S, Bjornsti MA, Houghton PJ . (2003). Rapamycins: mechanism of action and cellular resistance. Cancer Biol Ther 2: 222–232.
Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL et al. (2008). Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J 412: 211–221.
Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F et al. (2002). Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol 22: 7004–7014.
Ikeda Y, Sato K, Pimentel DR, Sam F, Shaw RJ, Dyck JR et al. (2009). Cardiac-specific deletion of LKB1 leads to hypertrophy and dysfunction. J Biol Chem 284: 35839–35849.
Inoki K, Ouyang H, Li Y, Guan KL . (2005). Signaling by target of rapamycin proteins in cell growth control. Microbiol Mol Biol Rev 69: 79–100.
Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X et al. (2006). TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126: 955–968.
Inoki K, Zhu T, Guan KL . (2003). TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590.
Jaleel M, McBride A, Lizcano JM, Deak M, Toth R, Morrice NA et al. (2005). Identification of the sucrose non-fermenting related kinase SNRK, as a novel LKB1 substrate. FEBS Lett 579: 1417–1423.
Jansen M, Ten Klooster JP, Offerhaus GJ, Clevers H . (2009). LKB1 and AMPK family signaling: the intimate link between cell polarity and energy metabolism. Physiol Rev 89: 777–798.
Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R et al. (1998). Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet 18: 38–43.
Jiang WG, Sampson J, Martin TA, Lee-Jones L, Watkins G, Douglas-Jones A et al. (2005). Tuberin and hamartin are aberrantly expressed and linked to clinical outcome in human breast cancer: the role of promoter methylation of TSC genes. Eur J Cancer 41: 1628–1636.
Jishage K, Nezu J, Kawase Y, Iwata T, Watanabe M, Miyoshi A et al. (2002). Role of Lkb1, the causative gene of Peutz-Jegher′s syndrome, in embryogenesis and polyposis. Proc Natl Acad Sci USA 99: 8903–8908.
Johannessen CM, Johnson BW, Williams SM, Chan AW, Reczek EE, Lynch RC et al. (2008). TORC1 is essential for NF1-associated malignancies. Curr Biol 18: 56–62.
Jozwiak J, Kotulska K, Lojek M, Galus R, Jozwiak S, Polnik D et al. (2009). Fibroblasts from normal skin of a tuberous sclerosis patient show upregulation of mTOR pathway. Am J Dermatopathol 31: 68–70.
Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J et al. (2009). ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 20: 1992–2003.
Kantidakis T, Ramsbottom BA, Birch JL, Dowding SN, White RJ . (2010). mTOR associates with TFIIIC, is found at tRNA and 5S rRNA genes, and targets their repressor Maf1. Proc Natl Acad Sci USA 107: 11823–11828.
Kasper M, Jaks V, Fiaschi M, Toftgard R . (2009). Hedgehog signalling in breast cancer. Carcinogenesis 30: 903–911.
Katajisto P, Vaahtomeri K, Ekman N, Ventela E, Ristimaki A, Bardeesy N et al. (2008). LKB1 signaling in mesenchymal cells required for suppression of gastrointestinal polyposis. Nat Genet 40: 455–459.
Kaufman McNamara E, Curtis AR, Fleischer Jr AB . (2010). Successful treatment of angiofibromata of tuberous sclerosis complex with rapamycin. J Dermatolog Treat (e-pub ahead of print).
Kenerson H, Dundon TA, Yeung RS . (2005). Effects of rapamycin in the Eker rat model of tuberous sclerosis complex. Pediatr Res 57: 67–75.
Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H et al. (2002). mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163–175.
Knowles MA, Hornigold N, Pitt E . (2003). Tuberous sclerosis complex (TSC) gene involvement in sporadic tumours. Biochem Soc Trans 31: 597–602.
Kobayashi T, Minowa O, Kuno J, Mitani H, Hino O, Noda T . (1999). Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line Tsc2 mutation in mice. Cancer Res 59: 1206–1211.
Koenig MK, Butler IJ, Northrup H . (2008). Regression of subependymal giant cell astrocytoma with rapamycin in tuberous sclerosis complex. J Child Neurol 23: 1238–1239.
Kola B, Boscaro M, Rutter GA, Grossman AB, Korbonits M . (2006). Expanding role of AMPK in endocrinology. Trends Endocrinol Metab 17: 205–215.
Krischock L, Beach R, Taylor J . (2010). Sirolimus and tuberous sclerosis-associated renal angiomyolipomas. Arch Dis Child 95: 391–392.
Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P et al. (2010). Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med 363: 1801–1811.
Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW . (1999). 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 48: 1667–1671.
Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N et al. (2002). A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet 11: 525–534.
Lam C, Bouffet E, Tabori U, Mabbott D, Taylor M, Bartels U . (2010). Rapamycin (sirolimus) in tuberous sclerosis associated pediatric central nervous system tumors. Pediatr Blood Cancer 54: 476–479.
Law BK . (2005). Rapamycin: an anti-cancer immunosuppressant? Crit Rev Oncol Hematol 56: 47–60.
Lee L, Sudentas P, Dabora SL . (2006). Combination of a rapamycin analog (CCI-779) and interferon-gamma is more effective than single agents in treating a mouse model of tuberous sclerosis complex. Genes Chromosomes Cancer 45: 933–944.
Lee L, Sudentas P, Donohue B, Asrican K, Worku A, Walker V et al. (2005). Efficacy of a rapamycin analog (CCI-779) and IFN-gamma in tuberous sclerosis mouse models. Genes Chromosomes Cancer 42: 213–227.
Lee PS, Tsang SW, Moses MA, Trayes-Gibson Z, Hsiao LL, Jensen R et al. (2010). Rapamycin-insensitive up-regulation of MMP2 and other genes in tuberous sclerosis complex 2-deficient lymphangioleiomyomatosis-like cells. Am J Respir Cell Mol Biol 42: 227–234.
Lim CT, Kola B, Korbonits M . (2010). AMPK as a mediator of hormonal signalling. J Mol Endocrinol 44: 87–97.
Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J et al. (2004). LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J 23: 833–843.
Massoumi R, Sjolander A . (2007). The role of leukotriene receptor signaling in inflammation and cancer. ScientificWorldJournal 7: 1413–1421.
McCarthy A, Lord CJ, Savage K, Grigoriadis A, Smith DP, Weigelt B et al. (2009). Conditional deletion of the Lkb1 gene in the mouse mammary gland induces tumour formation. J Pathol 219: 306–316.
McGarrity TJ, Amos C . (2006). Peutz-Jeghers syndrome: clinicopathology and molecular alterations. Cell Mol Life Sci 63: 2135–2144.
Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M et al. (2008). Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci 28: 5422–5432.
Mi R, Ma J, Zhang D, Li L, Zhang H . (2009). Efficacy of combined inhibition of mTOR and ERK/MAPK pathways in treating a tuberous sclerosis complex cell model. J Genet Genomics 36: 355–361.
Michels AA, Robitaille AM, Buczynski-Ruchonnet D, Hodroj W, Reina JH, Hall MN et al. (2010). mTORC1 directly phosphorylates and regulates human MAF1. Mol Cell Biol 30: 3749–3757.
Missiaglia E, Dalai I, Barbi S, Beghelli S, Falconi M, della Peruta M et al. (2010). Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol 28: 245–255.
Miyata H, Chiang AC, Vinters HV . (2004). Insulin signaling pathways in cortical dysplasia and TSC-tubers: tissue microarray analysis. Ann Neurol 56: 510–519.
Miyoshi H, Nakau M, Ishikawa TO, Seldin MF, Oshima M, Taketo MM . (2002). Gastrointestinal hamartomatous polyposis in Lkb1 heterozygous knockout mice. Cancer Res 62: 2261–2266.
Moore F, Weekes J, Hardie DG . (1991). Evidence that AMP triggers phosphorylation as well as direct allosteric activation of rat liver AMP-activated protein kinase. A sensitive mechanism to protect the cell against ATP depletion. Eur J Biochem 199: 691–697.
Muncy J, Butler IJ, Koenig MK . (2009). Rapamycin reduces seizure frequency in tuberous sclerosis complex. J Child Neurol 24: 477.
Onda H, Crino PB, Zhang H, Murphey RD, Rastelli L, Gould Rothberg BE et al. (2002). Tsc2 null murine neuroepithelial cells are a model for human tuber giant cells, and show activation of an mTOR pathway. Mol Cell Neurosci 21: 561–574.
Orlova KA, Crino PB . (2010). The tuberous sclerosis complex. Ann NY Acad Sci 1184: 87–105.
Ouyang J, Parakhia RA, Ochs RS . (2010). Metformin activates AMP-kinase through inhibition of AMP deaminase. J Biol Chem 286: 1–11.
Papanas N, Maltezos E, Mikhailidis DP . (2010). Metformin and cancer: licence to heal? Expert Opin Investig Drugs 19: 913–917.
Parrinello S, Lloyd AC . (2009). Neurofibroma development in NF1--insights into tumour initiation. Trends Cell Biol 19: 395–403.
Pearson HB, McCarthy A, Collins CM, Ashworth A, Clarke AR . (2008). Lkb1 deficiency causes prostate neoplasia in the mouse. Cancer Res 68: 2223–2232.
Peces R, Peces C, Cuesta-Lopez E, Perez-Duenas V, Vega-Cabrera C, Azorin S et al. (2010). Low-dose rapamycin reduces kidney volume angiomyolipomas and prevents the loss of renal function in a patient with tuberous sclerosis complex. Nephrol Dial Transplant 25: 3787–3791.
Podsypanina K, Lee RT, Politis C, Hennessy I, Crane A, Puc J et al. (2001). An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/− mice. Proc Natl Acad Sci USA 98: 10320–10325.
Pollizzi K, Malinowska-Kolodziej I, Stumm M, Lane H, Kwiatkowski D . (2009). Equivalent benefit of mTORC1 blockade and combined PI3K-mTOR blockade in a mouse model of tuberous sclerosis. Mol Cancer 8: 38.
Pressey JG, Wright JM, Geller JI, Joseph DB, Pressey CS, Kelly DR . (2010). Sirolimus therapy for fibromatosis and multifocal renal cell carcinoma in a child with tuberous sclerosis complex. Pediatr Blood Cancer 54: 1035–1037.
Rennebeck G, Kleymenova EV, Anderson R, Yeung RS, Artzt K, Walker CL . (1998). Loss of function of the tuberous sclerosis 2 tumor suppressor gene results in embryonic lethality characterized by disrupted neuroepithelial growth and development. Proc Natl Acad Sci USA 95: 15629–15634.
Robinson J, Lai C, Martin A, Nye E, Tomlinson I, Silver A . (2009). Oral rapamycin reduces tumour burden and vascularization in Lkb1(+/−) mice. J Pathol 219: 35–40.
Rosner M, Hanneder M, Siegel N, Valli A, Hengstschlager M . (2008). The tuberous sclerosis gene products hamartin and tuberin are multifunctional proteins with a wide spectrum of interacting partners. Mutat Res 658: 234–246.
Sancak O, Nellist M, Goedbloed M, Elfferich P, Wouters C, Maat-Kievit A et al. (2005). Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: genotype--phenotype correlations and comparison of diagnostic DNA techniques in tuberous sclerosis complex. Eur J Hum Genet 13: 731–741.
Sansal I, Sellers WR . (2004). The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol 22: 2954–2963.
Schwartz RA, Fernandez G, Kotulska K, Jozwiak S . (2007). Tuberous sclerosis complex: advances in diagnosis, genetics, and management. J Am Acad Dermatol 57: 189–202.
Sehgal SN, Baker H, Vezina C . (1975). Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 28: 727–732.
Shackelford DB, Shaw RJ . (2009). The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer 9: 563–575.
Shackelford DB, Vasquez DS, Corbeil J, Wu S, Leblanc M, Wu CL et al. (2009). mTOR and HIF-1alpha-mediated tumor metabolism in an LKB1 mouse model of Peutz-Jeghers syndrome. Proc Natl Acad Sci USA 106: 11137–11142.
Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA et al. (2004). The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 6: 91–99.
Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA et al. (2005). The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310: 1642–1646.
Sofer A, Lei K, Johannessen CM, Ellisen LW . (2005). Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol Cell Biol 25: 5834–5845.
Sparagana SP, Wilkes DC, Thompson CE, Bowers DC . (2010). Optic nerve tumor in tuberous sclerosis complex is not responsive to sirolimus. Pediatr Neurol 42: 443–446.
Spicer J, Rayter S, Young N, Elliott R, Ashworth A, Smith D . (2003). Regulation of the Wnt signalling component PAR1A by the Peutz-Jeghers syndrome kinase LKB1. Oncogene 22: 4752–4756.
Squarize CH, Castilho RM, Gutkind JS . (2008). Chemoprevention and treatment of experimental Cowden′s disease by mTOR inhibition with rapamycin. Cancer Res 68: 7066–7072.
Tarasewicz A, Debska-Slizien A, Konopa J, Zdrojewski Z, Rutkowski B . (2009). Rapamycin as a therapy of choice after renal transplantation in a patient with tuberous sclerosis complex. Transplant Proc 41: 3677–3682.
Tomlinson IP, Houlston RS . (1997). Peutz-Jeghers syndrome. J Med Genet 34: 1007–1011.
Uhlmann EJ, Li W, Scheidenhelm DK, Gau CL, Tamanoi F, Gutmann DH . (2004). Loss of tuberous sclerosis complex 1 (Tsc1) expression results in increased Rheb/S6K pathway signaling important for astrocyte cell size regulation. Glia 47: 180–188.
Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ et al. (2002). Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol 52: 285–296.
van Lier MG, Wagner A, van Leerdam ME, Biermann K, Kuipers EJ, Steyerberg EW et al. (2010). A review on the molecular diagnostics of Lynch syndrome: a central role for the pathology laboratory. J Cell Mol Med 14: 181–197.
van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S et al. (1997). Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277: 805–808.
van Slegtenhorst M, Nellist M, Nagelkerken B, Cheadle J, Snell R, van den Ouweland A et al. (1998). Interaction between hamartin and tuberin, the TSC1 and TSC2 gene products. Hum Mol Genet 7: 1053–1057.
Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH . (2007). Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9: 316–323.
Vezina C, Kudelski A, Sehgal SN . (1975). Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 28: 721–726.
Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C et al. (2008). Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology 135: 1972–1983, 1983.e1971-1911.
Wei C, Amos CI, Zhang N, Wang X, Rashid A, Walker CL et al. (2008). Suppression of Peutz-Jeghers polyposis by targeting mammalian target of rapamycin signaling. Clin Cancer Res 14: 1167–1171.
Wei C, Amos CI, Zhang N, Zhu J, Wang X, Frazier ML . (2009). Chemopreventive efficacy of rapamycin on Peutz-Jeghers syndrome in a mouse model. Cancer Lett 277: 149–154.
Wicker LS, Boltz Jr RC, Matt V, Nichols EA, Peterson LB, Sigal NH . (1990). Suppression of B cell activation by cyclosporin A, FK506 and rapamycin. Eur J Immunol 20: 2277–2283.
Wienecke R, Fackler I, Linsenmaier U, Mayer K, Licht T, Kretzler M . (2006). Antitumoral activity of rapamycin in renal angiomyolipoma associated with tuberous sclerosis complex. Am J Kidney Dis 48: e27–e29.
Wienecke R, Konig A, DeClue JE . (1995). Identification of tuberin, the tuberous sclerosis-2 product. Tuberin possesses specific Rap1GAP activity. J Biol Chem 270: 16409–16414.
Woodrum C, Nobil A, Dabora SL . (2010). Comparison of three rapamycin dosing schedules in A/J Tsc2+/− mice and improved survival with angiogenesis inhibitor or asparaginase treatment in mice with subcutaneous tuberous sclerosis related tumors. J Transl Med 8: 14.
Xiao GH, Shoarinejad F, Jin F, Golemis EA, Yeung RS . (1997). The tuberous sclerosis 2 gene product, tuberin, functions as a Rab5 GTPase activating protein (GAP) in modulating endocytosis. J Biol Chem 272: 6097–6100.
Yalon M, Ben-Sira L, Constantini S, Toren A . (2010). Regression of subependymal giant cell astrocytomas with RAD001 (Everolimus) in tuberous sclerosis complex. Childs Nerv Syst 27: 179–181.
Yeung RS . (2004). Lessons from the Eker rat model: from cage to bedside. Curr Mol Med 4: 799–806.
Ylikorkala A, Rossi DJ, Korsisaari N, Luukko K, Alitalo K, Henkemeyer M et al. (2001). Vascular abnormalities and deregulation of VEGF in Lkb1-deficient mice. Science 293: 1323–1326.
Yoo LI, Chung DC, Yuan J . (2002). LKB1—a master tumour suppressor of the small intestine and beyond. Nat Rev Cancer 2: 529–535.
Zeng LH, Xu L, Gutmann DH, Wong M . (2008). Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol 63: 444–453.
Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N et al. (2003a). Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest 112: 1223–1233.
Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D . (2003b). Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol 5: 578–581.
Acknowledgements
We wish to acknowledge the financial support from the Foundation ‘Michelle’ as well as from the TOP-institute pharma scheme of the Dutch government for their studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
van Veelen, W., Korsse, S., van de Laar, L. et al. The long and winding road to rational treatment of cancer associated with LKB1/AMPK/TSC/mTORC1 signaling. Oncogene 30, 2289–2303 (2011). https://doi.org/10.1038/onc.2010.630
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.630
Keywords
This article is cited by
-
AMPK induces degradation of the transcriptional repressor PROX1 impairing branched amino acid metabolism and tumourigenesis
Nature Communications (2022)
-
LKB1-PTEN axis controls Th1 and Th17 cell differentiation via regulating mTORC1
Journal of Molecular Medicine (2021)
-
Melatonin and neuroblastoma: a novel therapeutic approach
Molecular Biology Reports (2021)
-
Metformin effectively treats Tsc1 deletion-caused kidney pathology by upregulating AMPK phosphorylation
Cell Death Discovery (2020)
-
LGR5 marks targetable tumor-initiating cells in mouse liver cancer
Nature Communications (2020)