Abstract
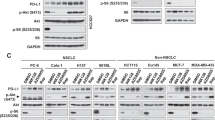
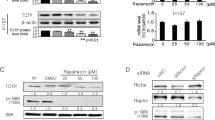
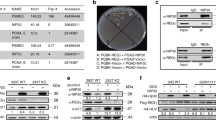
Acquisition of a transformed phenotype involves deregulation of several signal transduction pathways contributing to unconstrained cell growth. Understanding the interplay of different cancer-related signaling pathways is important for development of efficacious multitargeted anticancer drugs. The small molecule 9-aminoacridine (9AA) and its derivative, the antimalaria drug quinacrine, have selective toxicity for tumor cells and can simultaneously suppress nuclear factor-κB (NF-κB) and activate p53 signaling. To investigate the mechanism underlying these drug activities, we used a combination of two-dimensional protein separation by gel electrophoresis and mass spectrometry to identify proteins whose expression is altered in tumor cells by 9AA treatment. We found that 9AA treatment results in selective downregulation of a specific catalytic subunit of the phosphoinositide 3-kinase (PI3K) family, p110γ. Further exploration of this observation demonstrated that the mechanism of action of 9AA involves inhibition of the prosurvival AKT/mammalian target of rapamycin (mTOR) pathway that lies downstream of PI3K. p110γ translation appears to be regulated by mTOR and feeds back to further modulate mTOR and AKT, thereby impacting the p53 and NF-κB pathways as well. These results reveal functional interplay among the PI3K/AKT/mTOR, p53 and NF-κB pathways that are frequently deregulated in cancer and suggest that their simultaneous targeting by a single small molecule such as 9AA could result in efficacious and selective killing of transformed cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Bensaad K, Vousden KH . (2005). Savior and slayer: the two faces of p53. Nat Med 11: 1278–1279.
Budanov AV, Karin M . (2008). p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134: 451–460.
Camps M, Rückle T, Ji H, Ardissone V, Rintelen F, Shaw J et al. (2005). Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med 11: 936–943.
Furuta M, Weil RJ, Vortmeyer AO, Huang S, Lei J, Huang TN et al. (2004). Protein patterns and proteins that identify subtypes of glioblastoma multiforme. Oncogene 23: 6806–6814.
Feng Z, Zhang H, Levine AJ, Jin S . (2005). The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci USA 102: 8204–8209.
Gingras AC, Raught B, Sonenberg N . (2004). mTOR signaling to translation. Curr Top Microbiol Immunol 279: 169–197.
Gudkov AV . (2005). Therapeutic strategies based on pharmacological modulation of p53 pathway. In: Zambetti GP (ed). The p53 Tumor Suppressor Pathway and Cancer, vol. 2. Springer: New York.
Gurova KV, Hill JE, Guo C, Prokvolit A, Burdelya LG, Samoylova E et al. (2005). Small molecules that reactivate p53 in renal cell carcinoma reveal a NF-kappaB-dependent mechanism of p53 suppression in tumors. Proc Natl Acad Sci USA 102: 17448–17453.
Huang S, Bjornsti MA, Hounghton PJ . (2003). Rapamycins: mechanism of action and cellular resistance. Cancer Biol Ther 2: 222–232.
Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN . (2005). Activated AKT regulates NF-kappaB activation, p53 inhibition and cell survival in HTLV-1-transformed cells. Oncogene 24: 6719–6728.
Jung KJ, Dasgupta A, Huang K, Jeong SJ, Pise-Masison C, Gurova KV et al. (2008). Small-molecule inhibitor which reactivates p53 in human T-cell leukemia virus type 1-transformed cells. J Virol 82: 8537–8547.
Kang S, Denley A, Vanhaesebroeck B, Vogt PK . (2006). Oncogenic transformation induced by the p110β, -γ, and -δ isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci USA 103: 1289–1294.
Karin M, Gretin FR . (2005). NF-kB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5: 749–759.
Komar AA, Hatzoglou M . (2005). Internal ribosome entry sites in cellular mRNAs: mystery of their existence. J Biol Chem 280: 23425–23428.
Kucharczak J, Simmons MJ, Fan Y, Gelinas C . (2003). To be, or not to be: NF-kappaB is the answer. Role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene 22: 8961–8982.
Levine AJ, Feng ZH, Mak TW, You H, Jin SK . (2006). Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev 20: 267–275.
Mayo LD, Donner DB . (2001). Phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA 98: 11598–11603.
Mayo LD, Dixon JE, Durden DL, Tonks NK, Donner DB . (2002). PTEN protects p53 from mdm2 and sensitizes cancer cells to chemotherapy. J Biol Chem 277: 5484–5489.
Okamoto H, Li J, Glasker S, Vortmeyer AO, Jaffer H, Robinson RA et al. (2007). Proteomic comparison of oligodendrogliomas with and without 1pLOH. Cancer Biol and Ther 6: 391–396.
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB . (1999). NFkappaB activation by tumour necrosis factor requires the Akt serine–threonine kinase. Nature 401: 82–85.
Rommel C, Camps M, Ji H . (2007). PI3Kθ and PI3Kγ: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol 7: 191–201.
Rückle T, Schwarz MK, Rommel C . (2006). PI3Kγ inhibition: towards an ‘aspirin of the 21st century’? Nat Rev Drug Discov 5: 903–918.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM . (2005). Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science 307: 1098–1101.
Sizemore N, Lerner N, Dombrowski N, Sakurai H, Stark GR . (2002). Distuinct roles of the IkB kinase alpha and beta subunits in literating nuclear factor kB (NF-kB) from IkB and in phosphorylating the p53 subunit of NF-kB. J Biol Chem 277: 3863–3869.
Shi Y, Sharma A, Wu H, Lichtenstein A, Gera J . (2005). Cyclin D1 and c-myc internal ribosome entry site (IRES)-dependent translation is regulated by AKT activity and enhanced by rapamycin through a p38 MAPK- and ERK-dependent pathway. J Biol Chem 280: 10964–10973.
Stephens L, Smrcka A, Cooke FT, Jackson TR, Sternweis PC, Hawkins PT . (1994). A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein βγ subunits. Cell 77: 83–93.
Stone KL, Williams KR . (1993). Enzymatic digestion of proteins and HPLC peptide isolation. In: Matsudaira P (ed). A Practical Guide to Protein and Peptide Purification for Microsequencing. Academic Press: San Diego.
Terada N, Patel HR, Takase K, Kohno K, Nairn AC, Gelfand EW . (1994). Rapamycin selectively inhibits translation of mRNAs encoding elongation factors and ribosomal proteins. Proc Natl Acad Sci USA 91: 11477–11481.
Yamboliev IA, Wiesmann KM, Singer CA, Hedges JC, Gerthoffer WT . (2000). Phosphatidylinositol 3-kinases regulate ERK and p38 MAP kinases in canine colonic smooth muscle. Am J Physiol Cell Physiol 279: C352–C360.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Guo, C., Gasparian, A., Zhuang, Z. et al. 9-Aminoacridine-based anticancer drugs target the PI3K/AKT/mTOR, NF-κB and p53 pathways. Oncogene 28, 1151–1161 (2009). https://doi.org/10.1038/onc.2008.460
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.460
Keywords
This article is cited by
-
The drug efficacy testing in 3D cultures platform identifies effective drugs for ovarian cancer patients
npj Precision Oncology (2023)
-
Talazoparib enhances the quinacrine-mediated apoptosis in patient-derived oral mucosa CSCs by inhibiting BER pathway through the modulation of GCN5 and P300
Medical Oncology (2023)
-
Ipatasertib, a novel Akt inhibitor, induces transcription factor FoxO3a and NF-κB directly regulates PUMA-dependent apoptosis
Cell Death & Disease (2018)
-
Anti-survivin effect of the small molecule inhibitor YM155 in RCC cells is mediated by time-dependent inhibition of the NF-κB pathway
Scientific Reports (2018)
-
Inhibition of post-transcriptional steps in ribosome biogenesis confers cytoprotection against chemotherapeutic agents in a p53-dependent manner
Scientific Reports (2017)