Abstract
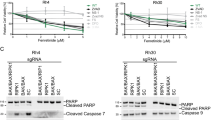
The Ewing's sarcoma family of tumours (ESFT) are small round cell tumours characterized by the non-random EWS–ETS gene rearrangements. We have previously demonstrated that ESFT are highly sensitive to fenretinide-induced death, effected in part through a reactive oxygen species (ROS)-dependent pathway. Here, we demonstrate for the first time that the sensitivity of ESFT cells to fenretinide-induced cell death is decreased following downregulation of the oncogenic fusion protein EWS-Fli1; siRNA targeting EWS-Fli1 attenuated fenretinide-induced cell death in cell lines expressing EWS-Fli1, but not EWS-ERG. This decrease in cell death was independent of the level of ROS produced following exposure to fenretinide, but was effected through EWS-Fli1-dependent modulation of p38MAPK activity. Furthermore, inhibition of p38MAPK activity and knockdown of EWS-Fli1 reduced fenretinide-induced mitochondrial permeabilization, cytochrome c release, caspase and PARP cleavage, consistent with the hypothesis that p38MAPK is critical for activation of the death cascade by fenretinide in ESFT cells. These data demonstrate that expression of EWS–Fli1 enhances fenretinide-induced cell death in ESFT and that this is effected at least in part through modulation of p38MAPK activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Asumendi A, Morales MC, Alvarez A, Arechaga J, Perez-Yarza G . (2002). Implication of mitochondria-derived ROS and cardiolipin peroxidation in N-(4-hydroxyphenyl)retinamide-induced apoptosis. Br J Cancer 86: 1951–1956.
Benhar M, Dalyot I, Engelberg D, Levitzki A . (2001). Enhanced ROS production in oncogenically transformed cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress. Mol Cell Biol 21: 6913–6926.
Boya P, Morales MC, Gonzalez-Polo RA, Andreau K, Gourdier I, Perfettini JL et al. (2003). The chemopreventive agent N-(4-hydroxyphenyl)retinamide induces apoptosis through a mitochondrial pathway regulated by proteins from the Bcl-2 family. Oncogene 22: 6220–6230.
Burchill SA, Bradbury FM, Smith B, Lewis IJ, Selby P . (1994). Neuroblastoma cell detection by reverse transcriptase-polymerase chain reaction (RT–PCR) for tyrosine hydroxylase mRNA. Int J Cancer 57: 671–675.
Burchill SA . (2003). Ewing's sarcoma: diagnostic, prognostic, and therapeutic implications of molecular abnormalities. J Clin Pathol 56: 96–102.
Castillero-Trejo Y, Eliazer S, Xiang L, Richardson JA, Ilaria Jr RL . (2005). Expression of the EWS/FLI-1 oncogene in murine primary bone-derived cells results in EWS/FLI-1-dependent, Ewing sarcoma-like tumors. Cancer Res 65: 8698–8705.
Chansky HA, Barahmand-Pour F, Mei Q, Kahn-Farooqi W, Zielinska-Kwiatkowska A, Blackburn M et al. (2004). Targeting of EWS/FLI-1 by RNA interference attenuates the tumor phenotype of Ewing's sarcoma cells in vitro. J Orthop Res 22: 910–917.
Chiesa F, Tradati N, Grigolato R, Boracchi P, Biganzoli E, Crose N et al. (2005). Randomized trial of fenretinide (4-HPR) to prevent recurrences, new localizations and carcinomas in patients operated on for oral leukoplakia: long-term results. Int J Cancer 115: 625–629.
Corazzari M, Lovat PE, Oliverio S, Di Sano F, Donnorso RP, Redfern CP et al. (2005). Analysis of mitochondria during cell death. Biochem Biophys Res Commun 331: 810–815.
Delattre O, Zucman J, Melot T, Garau XS, Zucker JM, Lenoir GM et al. (1994). The Ewing family of tumors-a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med 331: 294–299.
Dohjima T, Lee NS, Li H, Ohno T, Rossi JJ . (2003). Small interfering RNAs expressed from a Pol III promoter suppress the EWS/Fli-1 transcript in a Ewing sarcoma cell line. Mol Ther 7: 811–816.
Formelli F, Clerici M, Campa T, Di Mauro MG, Magni A, Mascotti G et al. (1993). Synthetic retinoid fenretinide is effective against a human ovarian carcinoma xenograft and potentiates cisplatin activity. J Clin Oncol 11: 2036–2042.
Garaventa A, Luksch R, Lo Piccolo MS, Cavadini E, Montaldo PG, Pizzitola MR et al. (2003). Phase I trial and pharmacokinetics of fenretinide in children with neuroblastoma. Clin Cancer Res 9: 2032–2039.
Horbinski C, Chu CT . (2005). Kinase signaling cascades in the mitochondrion: a matter of life or death. Free Radic Biol Med 38: 2–11.
Huang S, Shu L, Easton J, Harwood FC, Germain GS, Ichijo H et al. (2004). Inhibition of mammalian target of rapamycin activates apoptosis signal-regulating kinase 1 signaling by suppressing protein phosphatase 5 activity. J Biol Chem 279: 36490–36496.
Hu-Lieskovan S, Heidel JD, Bartlett DW, Davis ME, Triche TJ . (2005b). Sequence-specific knock-down of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing's sarcoma. Cancer Res 65: 8984–8992.
Hu-Lieskovan S, Zhang J, Wu L, Shimada H, Schofield DE, Triche TJ . (2005a). EWS-FLI1 fusion protein up-regulates critical genes in neural crest development and is responsible for the observed phenotype of Ewing's family of tumors. Cancer Res 65: 4633–4644.
Johnson GL, Lapadat R . (2002). Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298: 1911–1912.
Kim HJ, Chakravarti N, Oridate N, Choe C, Claret FX, Lotan R . (2006). N-(4-Hydroxyphenyl) retinamide-induced apoptosis triggered by reactive oxygen species is mediated by activation of MAPKs in head and neck squamous carcinoma cells. Oncogene 25: 2785–2794.
Malone W, Perloff M, Crowell J, Sigman C, Higley H . (2003). Fenretinide: a prototype cancer prevention drug. Expert Opin Investig Drugs 12: 1829–1842.
Matsunobu T, Tanaka K, Nakamura T, Nakatani F, Sakimura R, Hanada M et al. (2006). The possible role of EWS-Fli1 in evasion of senescence in Ewing family tumors. Cancer Res 66: 803–811.
May WA, Gishizky ML, Lessnick SL, Lunsford LB, Lewis BC, Delattre O et al. (1993). Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc Natl Acad Sci USA 90: 5752–5756.
Mittal V . (2004). Improving the efficiency of RNA interference in mammals. Nat Rev Genet 5: 355–365.
Myatt SS, Redfern CP, Burchill SA . (2005). p38MAPK-Dependent sensitivity of Ewing's sarcoma family of tumors to fenretinide-induced cell death. Clin Cancer Res 11: 3136–3148.
O’Donnell PH, Guo WX, Reynolds CP, Maurer BJ . (2002). N-(4-hydroxyphenyl)retinamide increases ceramide and is cytotoxic to acute lymphoblastic leukemia cell lines, but not to non-malignant lymphocytes. Leukemia 16: 902–910.
Osone S, Hosoi H, Kuwahara Y, Matsumot Y, Lehara T, Sugimoto T . (2004). Fenretinide induces sustained-activation of JNK/p38 MAPK and apoptosis in a reactive oxygen species-dependent manner in neuroblastoma cells. Int J Cancer 112: 219–224.
Pienta KJ, Esper PS, Zwas F, Krzeminski R, Flaherty LE . (1997). Phase II chemoprevention trial of oral fenretinide in patients at risk for adenocarcinoma of the prostate. Am J Clin Oncol 20: 36–39.
Prieur A, Tirode F, Cohen P, Delattre O . (2004). EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol Cell Biol 24: 7275–7283.
Simeone AM, Deng CX, Kelloff GJ, Steele VE, Johnson MM, Tari AM . (2005). N-(4-Hydroxyphenyl)retinamide is more potent than other phenylretinamides in inhibiting the growth of BRCA1-mutated breast cancer cells. Carcinogenesis 26: 1000–1007.
Smith R, Owen LA, Trem DJ, Wong JS, Whangbo JS, Golub TR et al. (2006). Expression profiling of EWS/FLI identifies NKX2.2 as a critical target gene in Ewing's sarcoma. Cancer Cell 9: 405–416.
Takeda K, Matsuzawa A, Nishitoh H, Ichijo H . (2003). Roles of MAPKKK ASK1 in stress-induced cell death. Cell Struct Funct 28: 23–29.
Thompson AD, Teitell MA, Arvand A, Denny CT . (1999). Divergent Ewing's sarcoma EWS/ETS fusions confer a common tumorigenic phenotype on NIH3T3 cells. Oncogene 18: 5506–5513.
Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K et al. (2001). ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2: 222–228.
Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J . (2002). Redox control of cell death. Antioxid Redox Signal 4: 405–414.
Villablanca JG, Krailo MD, Ames MM, Reid JM, Reaman GH, Reynolds CP . (2006). Phase I trial of oral fenretinide in children with high-risk solid tumors: a report from the Children's Oncology Group (CCG 09709). J Clin Oncol 24: 3423–3430.
Westerhout EM, Ooms M, Vink M, Das AT, Berkhout B . (2005). HIV-1 can escape from RNA interference by evolving an alternative structure in its RNA genome. Nucleic Acids Res 33: 796–804.
Williamson AJ, Dibling BC, Boyne JR, Selby P, Burchill SA . (2004). Basic fibroblast growth factor-induced cell death is effected through sustained activation of p38MAPK and up-regulation of the death receptor p75NTR. J Biol Chem 279: 47912–47928.
Won JS, Singh I . (2006). Sphingolipid signaling and redox regulation. Free Radic Biol Med 40: 1875–1888.
Wu JM, DiPietrantonio AM, Hsieh TC . (2001). Mechanism of fenretinide (4-HPR)-induced cell death. Apoptosis 6: 377–388.
Yi H, Fujimura Y, Ouchida M, Prasad DD, Rao VN, Reddy ES . (1997). Inhibition of apoptosis by normal and aberrant Fli-1 and erg proteins involved in human solid tumors and leukemias. Oncogene 14: 1259–1268.
Zanardi S, Serrano D, Argusti A, Barile M, Puntoni M, Decensi A . (2006). Clinical trials with retinoids for breast cancer chemoprevention. Endocr Relat Cancer 13: 51–68.
Acknowledgements
Thank you to Mr Colin Johnston for statistical advice, and to Mrs Andrea Berry for technical assistance. This work was supported by the Candlelighter's Trust, United Kingdom (SAB), MJP Cancer Care Trust Fund, United Kingdom (SAB) and University of Leeds Research Scholarship (SSM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Myatt, S., Burchill, S. The sensitivity of the Ewing's sarcoma family of tumours to fenretinide-induced cell death is increased by EWS-Fli1-dependent modulation of p38MAPK activity. Oncogene 27, 985–996 (2008). https://doi.org/10.1038/sj.onc.1210705
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210705
Keywords
This article is cited by
-
RNA sequencing and functional studies of patient-derived cells reveal that neurexin-1 and regulators of this pathway are associated with poor outcomes in Ewing sarcoma
Cellular Oncology (2021)
-
Optimal Management of Ewing Sarcoma Family of Tumors: Recent Developments in Systemic Therapy
Pediatric Drugs (2013)
-
Detection and characterisation of multi-drug resistance protein 1 (MRP-1) in human mitochondria
British Journal of Cancer (2012)
-
GSTM4 is a microsatellite-containing EWS/FLI target involved in Ewing's sarcoma oncogenesis and therapeutic resistance
Oncogene (2009)