Abstract
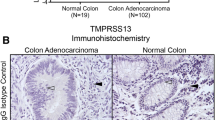
TMPRSS4 is a novel type II transmembrane serine protease found at the cell surface that is highly expressed in pancreatic, colon and gastric cancer tissues. However, the biological functions of TMPRSS4 in cancer are unknown. Here we show, using reverse transcription–PCR, that TMPRSS4 is highly elevated in lung cancer tissues compared with normal tissues and is also broadly expressed in a variety of human cancer cell lines. Knockdown of TMPRSS4 by small interfering RNA treatment in lung and colon cancer cell lines was associated with reduction of cell invasion and cell-matrix adhesion as well as modulation of cell proliferation. Conversely, the invasiveness, motility and adhesiveness of SW480 colon carcinoma cells were significantly enhanced by TMPRSS4 overexpression. Furthermore, overexpression of TMPRSS4 induced loss of E-cadherin-mediated cell–cell adhesion, concomitant with the induction of SIP1/ZEB2, an E-cadherin transcriptional repressor, and led to epithelial–mesenchymal transition events, including morphological changes, actin reorganization and upregulation of mesenchymal markers. TMPRSS4-overexpressing cells also displayed markedly increased metastasis to the liver in nude mice upon intrasplenic injection. Taken together, these studies suggest that TMPRSS4 controls the invasive and metastatic potential of human cancer cells by facilitating an epithelial–mesenchymal transition; TMPRSS4 may be a potential therapeutic target for cancer treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Andreasen PA, Kjoller L, Christensen L, Duffy MJ . (1997). The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 72: 1–22.
Aplin AE, Howe A, Alahari SK, Juliano RL . (1998). Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev 50: 197–263.
Benaud CM, Oberst M, Dickson RB, Lin CY . (2002). Deregulated activation of matriptase in breast cancer cells. Clin Exp Metast 19: 639–649.
Berntzen G, Lunde E, Flobakk M, Andersen JT, Lauvrak V, Sandlie I . (2005). Prolonged and increased expression of soluble Fc receptors, IgG and a TCR-Ig fusion protein by transiently transfected adherent 293E cells. J Immunol Methods 298: 93–104.
Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA et al (2001). Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA 98: 10356–10361.
Braga VM . (2002). Cell–cell adhesion and signalling. Curr Opin Cell Biol 14: 546–556.
Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E et al (2001). The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell 7: 1267–1278.
Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze'ev A . (2003). Autoregulation of E-cadherin expression by cadherin–cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK. J Cell Biol 163: 847–857.
Del Rosso M, Fibbi G, Pucci M, D'Alessio S, Del Rosso A, Magnelli L et al (2002). Multiple pathways of cell invasion are regulated by multiple families of serine proteases. Clin Exp Metast 19: 193–207.
Deryugina EI, Quigley JP . (2006). Matrix metalloproteinases and tumor metastasis. Cancer Metast Rev 25: 9–34.
Duffy MJ . (1996). Proteases as prognostic markers in cancer. Clin Cancer Res 2: 613–618.
Duffy MJ . (2002). Urokinase-type plasminogen activator: a potent marker of metastatic potential in human cancers. Biochem Soc Trans 30: 207–210.
Egeblad M, Werb Z . (2002). New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2: 161–174.
Feltes CM, Kudo A, Blaschuk O, Byers SW . (2002). An alternatively spliced cadherin-11 enhances human breast cancer cell invasion. Cancer Res 62: 6688–6697.
Forbs D, Thiel S, Stella MC, Sturzebecher A, Schweinitz A, Steinmetzer T et al (2005). In vitro inhibition of matriptase prevents invasive growth of cell lines of prostate and colon carcinoma. Int J Oncol 27: 1061–1070.
Galkin AV, Mullen L, Fox WD, Brown J, Duncan D, Moreno O et al (2004). CVS-3983, a selective matriptase inhibitor, suppresses the growth of androgen independent prostate tumor xenografts. Prostate 61: 228–235.
Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA . (2000). Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol 148: 779–790.
Hooper JD, Clements JA, Quigley JP, Antalis TM . (2001). Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. J Biol Chem 276: 857–860.
Huang X, Chen S, Xu L, Liu Y, Deb DK, Platanias LC et al (2005). Genistein inhibits p38 map kinase activation, matrix metalloproteinase type 2, and cell invasion in human prostate epithelial cells. Cancer Res 65: 3470–3478.
Kebebew E, Peng M, Reiff E, Duh QY, Clark OH, McMillan A . (2005). ECM1 and TMPRSS4 are diagnostic markers of malignant thyroid neoplasms and improve the accuracy of fine needle aspiration biopsy. Ann Surg 242: 353–361; discussion 361–363.
Kim S, Bakre M, Yin H, Varner JA . (2002). Inhibition of endothelial cell survival and angiogenesis by protein kinase A. J Clin Invest 110: 933–941.
Laferriere J, Houle F, Huot J . (2002). Regulation of the metastatic process by E-selectin and stress-activated protein kinase-2/p38. Ann NY Acad Sci 973: 562–572.
Lin CY, Anders J, Johnson M, Sang QA, Dickson RB . (1999). Molecular cloning of cDNA for matriptase, a matrix-degrading serine protease with trypsin-like activity. J Biol Chem 274: 18231–18236.
List K, Bugge TH, Szabo R . (2006). Matriptase: potent proteolysis on the cell surface. Mol Med 12: 1–7.
List K, Szabo R, Molinolo A, Sriuranpong V, Redeye V, Murdock T et al (2005). Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev 19: 1934–1950.
Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ . (1997). Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 139: 1861–1872.
Maschler S, Wirl G, Spring H, Bredow DV, Sordat I, Beug H et al (2005). Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene 24: 2032–2041.
Mignatti P, Rifkin DB . (1993). Biology and biochemistry of proteinases in tumor invasion. Physiol Rev 73: 161–195.
Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM . (2000). Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol 18: 1135–1149.
Netzel-Arnett S, Hooper JD, Szabo R, Madison EL, Quigley JP, Bugge TH et al (2003). Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metast Rev 22: 237–258.
Nobes CD, Hall A . (1995). Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81: 53–62.
Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE et al (2005). Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436: 123–127.
Srikantan V, Valladares M, Rhim JS, Moul JW, Srivastava S . (2002). HEPSIN inhibits cell growth/invasion in prostate cancer cells. Cancer Res 62: 6812–6816.
Stetler-Stevenson WG, Yu AE . (2001). Proteases in invasion: matrix metalloproteinases. Semin Cancer Biol 11: 143–152.
Suzuki M, Kobayashi H, Kanayama N, Saga Y, Suzuki M, Lin CY et al (2004). Inhibition of tumor invasion by genomic down-regulation of matriptase through suppression of activation of receptor-bound pro-urokinase. J Biol Chem 279: 14899–14908.
Szabo R, Wu Q, Dickson RB, Netzel-Arnett S, Antalis TM, Bugge TH . (2003). Type II transmembrane serine proteases. Thromb Haemost 90: 185–193.
Takeuchi T, Shuman MA, Craik CS . (1999). Reverse biochemistry: use of macromolecular protease inhibitors to dissect complex biological processes and identify a membrane-type serine protease in epithelial cancer and normal tissue. Proc Natl Acad Sci USA 96: 11054–11061.
Thiery JP . (2002). Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer 2: 442–454.
Thiery JP, Sleeman JP . (2006). Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol 7: 131–142.
Thompson EW, Newgreen DF, Tarin D . (2005). Carcinoma invasion and metastasis: a role for epithelial–mesenchymal transition? Cancer Res 65: 5991–5995; discussion 5995.
van Hengel J, van Roy F . (2007). Diverse functions of p120ctn in tumors. Biochim Biophys Acta 1773: 78–88.
Vandewalle C, Comijn J, De Craene B, Vermassen P, Bruyneel E, Andersen H et al (2005). SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell–cell junctions. Nucleic Acids Res 33: 6566–6578.
Wallrapp C, Hahnel S, Muller-Pillasch F, Burghardt B, Iwamura T, Ruthenburger M et al (2000). A novel transmembrane serine protease (TMPRSS3) overexpressed in pancreatic cancer. Cancer Res 60: 2602–2606.
Xuan JA, Schneider D, Toy P, Lin R, Newton A, Zhu Y et al (2006). Antibodies neutralizing hepsin protease activity do not impact cell growth but inhibit invasion of prostate and ovarian tumor cells in culture. Cancer Res 66: 3611–3619.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, H., Lee, K., Park, S. et al. TMPRSS4 promotes invasion, migration and metastasis of human tumor cells by facilitating an epithelial–mesenchymal transition. Oncogene 27, 2635–2647 (2008). https://doi.org/10.1038/sj.onc.1210914
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210914
Keywords
This article is cited by
-
TMPRSS4, a type II transmembrane serine protease, as a potential therapeutic target in cancer
Experimental & Molecular Medicine (2023)
-
MicroRNAs as the critical regulators of cell migration and invasion in thyroid cancer
Biomarker Research (2022)
-
Utility of TMPRSS4 as a Prognostic Biomarker and Potential Therapeutic Target in Patients with Gastric Cancer
Journal of Gastrointestinal Surgery (2022)
-
TMPRSS4 promotes cancer stem–like properties in prostate cancer cells through upregulation of SOX2 by SLUG and TWIST1
Journal of Experimental & Clinical Cancer Research (2021)
-
Identification of a novel synthetic lethal vulnerability in non-small cell lung cancer by co-targeting TMPRSS4 and DDR1
Scientific Reports (2019)