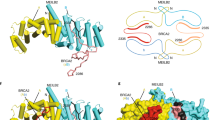
Abstract
Homologous recombination has a dual role in eukaryotic organisms. Firstly, it is responsible for the creation of genetic variability during meiosis by directing the formation of reciprocal crossovers that result in random combinations of alleles and traits. Secondly, in mitotic cells, it maintains the integrity of the genome by promoting the faithful repair of DNA double-strand breaks (DSBs). In vertebrates, it therefore plays a key role in tumour avoidance. Mutations in the tumour suppressor protein BRCA2 are associated with predisposition to breast and ovarian cancers, and loss of BRCA2 function leads to genetic instability. BRCA2 protein interacts directly with the RAD51 recombinase and regulates recombination-mediated DSB repair, accounting for the high levels of spontaneous chromosomal aberrations seen in BRCA2-defective cells. Recent observations indicate that BRCA2 also plays a critical role in meiotic recombination, this time through direct interactions with the meiosis-specific recombinase DMC1. The interactions of BRCA2 with RAD51 and DMC1 lead us to suggest that the BRCA2 tumour suppressor is a universal regulator of recombinase actions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Atanassov BS, Barrett JC, Davis BJ . (2005). Homozygous germ-line mutation in exon 27 of murine BRCA2 disrupts the FANCD2-BRCA2 pathway in the homologous recombination-mediated DNA interstrand cross-link repair but does not affect meiosis. Genes Chromosomes Cancer 44: 429–437.
Barlow AL, Benson FE, West SC, Hultén MA . (1997). Distribution of RAD51 recombinase in human and mouse spermatocytes. EMBO J 16: 5207–5215.
Bennett CB, Lewis AL, Baldwin KK, Resnick MA . (1993). Lethality induced by a single site-specific double-strand break in a dispensable yeast plasmid. Proc Natl Acad Sci USA 90: 5613–5617.
Benson FE, Stasiak A, West SC . (1994). Purification and characterisation of the human RAD51 protein, an analogue of E. coli RecA. EMBO J 13: 5764–5771.
Chen CF, Chen PL, Zhong Q, Sharp ZD, Lee WH . (1999). Expression of BRC repeats in breast cancer cells disrupts the BRCA2–RAD51 complex and leads to radiation hypersensitivity and loss of G(2)/M checkpoint control. J Biol Chem 274: 32931–32935.
Chen JJ, Silver DP, Walpita D, Cantor SB, Gazdar AF, Tomlinson G et al. (1998a). Stable interaction between the products of the BRCA1 and BRCA2 tumor suppressor genes in mitotic and meiotic cells. Mol Cell 2: 317–328.
Chen PL, Chen CF, Chen YM, Xiao J, Sharp ZD, Lee WH . (1998b). The BRC repeats in BRCA2 are critical for RAD51 binding and resistance to methyl methanesulfonate treatment. Proc Natl Acad Sci USA 95: 5287–5292.
Connor F, Bertwistle D, Mee PJ, Ross GM, Swift S, Grigorieva E et al. (1997). Tumorigenesis and a DNA-repair defect in mice with a truncating BRCA2 mutation. Nat Genet 17: 423–430.
Conway AB, Lynch TW, Zhang Y, Fortin GS, Fung CW, Symington LS et al. (2004). Crystal structure of a Rad51 filament. Nat Struct Mol Biol 11: 791–796.
Davies AA, Masson J-Y, McIlwraith MJ, Stasiak AZ, Stasiak A, Venkitaraman AR et al. (2001). Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol Cell 7: 273–282.
Davies OR, Pellegrini L . (2007). Interaction with the BRCA2 C-terminus protects RAD51-DNA filaments from disassembly by BRC repeats. Nat Struct Mol Biol 14: 475–483.
Donoho G, Brenneman MA, Cui TX, Donoviel D, Vogel H, Goodwin EH et al. (2003). Deletion of BRCA2 exon 27 causes hypersensitivity to DNA crosslinks, chromosomal instability, and reduced life span in mice. Genes Chromosomes Cancer 36: 317–331.
Dray E, Siaud N, Dubois E, Doutriaux MP . (2006). Interaction between Arabidopsis BRCA2 and its partners RAD51, DMC1, and DSS1. Plant Physiol 140: 1059–1069.
Esashi F, Christ N, Gannon J, Liu Y, Hunt T, Jasin M et al. (2005). CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature 434: 598–604.
Esashi F, Galkin VE, Yu X, Egelman EH, West SC . (2007). Stabilization of RAD51 nucleoprotein filaments by the C-terminal region of BRCA2. Nat Struct Mol Biol 14: 468–474.
Galkin VE, Esashi F, Yu X, Yang SX, West SC, Egelman EH . (2005). BRCA2 BRC motifs bind RAD51-DNA filaments. Proc Natl Acad Sci USA 102: 8537–8542.
Goggins M, Schutte M, Lu J, Moskaluk CA, Weinstein CL, Petersen GM et al. (1996). Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Can Res 56: 5360–5364.
Haaf T, Golub EI, Reddy G, Radding CM, Ward DC . (1995). Nuclear foci of mammalian RAD51 recombination protein in somatic cells after DNA damage and its localization in synaptonemal complexes. Proc Natl Acad Sci USA 92: 2298–2302.
Hoeijmakers JH . (2001). Genome maintenance mechanisms for preventing cancer. Nature 411: 366–374.
Kinebuchi T, Kagawa W, Enomoto R, Tanaka K, Miyagawa K, Shibata T et al. (2004). Structural basis for octameric ring formation and DNA interaction of the human homologous-pairing protein DMC1. Mol Cell 14: 363–374.
Kojic M, Kostrub CF, Buchman AR, Holloman WK . (2002). BRCA2 homolog required for proficiency in DNA repair, recombination, and genome stability in Ustilago maydis. Mol Cell 10: 683–691.
Lim DS, Hasty P . (1996). A mutation in mouse RAD51 results in an early embryonic lethal that is suppressed by a mutation in p53. Mol Cell Biol 16: 7133–7143.
Lindahl T . (1993). Instability and decay of the primary structure of DNA. Nature 362: 709–715.
Ludwig T, Chapman DL, Papaioannou VE, Efstratiadis A . (1997). Targeted mutations of breast cancer susceptibility gene homologs in mice: lethal phenotypes of BRCA1, BRCA2, BRCA1/BRCA2, BRCA1/p53, and BRCA2/p53 null zygous embryos. Genes Dev 11: 1226–1241.
Marmorstein LY, Ouchi T, Aaronson SA . (1998). The BRCA2 gene product functionally interacts with p53 and RAD51. Proc Natl Acad Sci USA 95: 13869–13874.
Martin JS, Winkelmann N, Petalcorin MIR, McIlwraith MJ, Boulton SJ . (2005). RAD-51-dependent and independent roles of a Caenorhabditis elegans BRCA2-related protein during DNA double-strand break repair. Mol Cell Biol 25: 3127–3139.
McAllister KA, Bennett LM, Houle CD, Ward TA, Malphurs J, Collins NK et al. (2002). Cancer susceptibility of mice with a homozygous deletion of the COOH-terminal domain of the BRCA2 gene. Cancer Res 62: 990–994.
Mizuta R, Lasalle JM, Cheng HL, Shinohara A, Ogawa H, Copeland N et al. (1997). Rab22 and Rab163/mouse BRCA2: proteins that specifically interact with the RAD51 protein. Proc Natl Acad Sci USA 94: 6927–6932.
Moynahan ME, Pierce AJ, Jasin M . (2001). BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell 7: 263–272.
Neale MJ, Keeney S . (2006). Clarifying the mechanics of DNA strand exchange in meiotic recombination. Nature 442: 153–158.
Patel KJ, Yu VPCC, Lee H, Corcoran A, Thistlethwaite FC, Evans MJ et al. (1998). Involvement of BRCA2 in DNA repair. Mol Cell 1: 347–357.
Pellegrini L, Yu DS, Lo T, Anand S, Lee MY, Blundell TL et al. (2002). Insights into DNA recombination from the structure of a RAD51–BRCA2 complex. Nature 420: 287–293.
Petalcorin MIR, Galkin VE, Yu X, Egelman EH, Boulton SJ . (2007). Stabilization of RAD-51-DNA filaments by a novel interaction domain in Caenorhabditis elegans BRCA2. Proc Natl Acad Sci USA 104: 8299–8304.
Pittman DL, Cobb J, Schimenti KJ, Wilson LA, Cooper DM, Brignull E et al. (1998). Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for DMC1, a germline-specific RecA homolog. Mol Cell 1: 697–705.
Raderschall E, Golub EI, Haaf T . (1999). Nuclear foci of mammalian recombination proteins are located at single-stranded DNA regions formed after DNA damage. Proc Natl Acad Sci USA 96: 1921–1926.
Saeki H, Siaud N, Christ N, Wiegant WW, van BPPW, Han MG et al. (2006). Suppression of the DNA repair defects of BRCA2-deficient cells with heterologous protein fusions. Proc Natl Acad Sci USA 103: 8768–8773.
Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J et al. (1997). Association of BRCA1 with RAD51 in mitotic and meiotic cells. Cell 88: 265–275.
Sehorn MG, Sigurdsson S, Bussen W, Unger VM, Sung P . (2004). Human meiotic recombinase DMC1 promotes ATP-dependent homologous DNA strand exchange. Nature 429: 433–437.
Sharan SK, Morimatsu M, Albrecht U, Lim SS, Regel E, Dinh C et al. (1997). Embryonic lethality and radiation hypersensitivity mediated by RAD51 in mice lacking BRCA2. Nature 386: 804–810.
Sharan SK, Pyle A, Coppola V, Babus J, Swaminathan S, Benedict J et al. (2004). BRCA2 deficiency in mice leads to meiotic impairment and infertility. Development 131: 131–142.
Shin DS, Pellegrini L, Daniels DS, Yelent B, Craig L, Bates D et al. (2003). Full-length archaeal RAD51 structure and mutants: mechanisms for RAD51 assembly and control by BRCA2. EMBO J 22: 4566–4576.
Shinohara M, Gasior SL, Bishop DK, Shinohara A . (2000). Tid1/Rdh54 promotes colocalization of Rad51 and Dmc1 during meiotic recombination. Proc Natl Acad Sci USA 97: 10814–10819.
Shivji MKK, Davies OR, Savill JM, Bates DL, Pellegrini L, Venkitaraman AR . (2006). A region of human BRCA2 containing multiple BRC repeats promotes RAD51-mediated strand exchange. Nucl Acids Res 34: 4000–4011.
Siaud N, Dray E, Gy I, Gerard E, Takvorian N, Doutriaux MP . (2004). BRCA2 is involved in meiosis in Arabidopsis thaliana as suggested by its interaction with DMC1. EMBO J 23: 1392–1401.
Sung P, Klein H . (2006). Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nat Rev Mol Cell Biol 7: 739–750.
Suzuki A, Delapompa JL, Hakem R, Elia A, Yoshida R, Mo R et al. (1997). BRCA2 is required for embryonic cellular proliferation in the mouse. Genes Dev 11: 1242–1252.
Takanami T, Mori A, Takahashi H, Higashitani A . (2000). Hyper-resistance of meiotic cells to radiation due to a strong expression of a single recA-like gene in Caenorhabditis elegans. Nucl Acids Res 28: 4232–4236.
Takata M, Tachiiri S, Fujimori A, Thompson LH, Miki F, Hiraoka M et al. (2002). Conserved domains in the chicken homologue of BRCA2. Oncogene 21: 1130–1134.
Tarsounas M, Davies AA, West SC . (2004). RAD51 localization and activation following DNA damage. Phil Trans R Soc Lond B 359: 87–93.
Tarsounas M, Morita T, Pearlman RE, Moens PB . (1999). RAD51 and DMC1 form mixed complexes associated with mouse meiotic chromosome cores and synaptonemal complexes. J Cell Biol 147: 207–219.
Thorslund T, Esashi F, West SC . (2007). Interactions between human BRCA2 protein and the meiosis-specific recombinase DMC1. EMBO J 26: 2915–2922.
Tsuzuki T, Fujii Y, Sakuma K, Tominaga Y, Nakao K, Sekiguchi M et al. (1996). Targeted disruption of the RAD51 gene leads to lethality in embryonic mice. Proc Natl Acad Sci USA 93: 6236–6240.
Tutt A, Bertwistle D, Valentine J, Gabriel A, Swift S, Ross G et al. (2001). Mutation in BRCA2 stimulates error-prone homology-directed repair of DNA double-strand breaks occurring between repeated sequences. EMBO J 20: 4704–4716.
West SC . (2003). Molecular views of recombination proteins and their control. Nat Rev Mol Cell Biol 4: 435–445.
Wong AKC, Pero R, Ormonde PA, Tavtigian SV, Bartel PL . (1997). RAD51 interacts with the evolutionarily conserved BRC motifs in the human breast cancer susceptibility gene BRCA2. J Biol Chem 272: 31941–31944.
Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J et al. (1995). Identification of the breast cancer susceptibility gene BRCA2. Nature 378: 789–792.
Yang H, Li Q, Holloman WK, Pavletich NP . (2005). The BRCA2 homologue BRH2 nucleates RAD51 filament formation at a dsDNS–ssDNA junction. Nature 433: 653–657.
Yang HJ, Jeffrey PD, Miller J, Kinnucan E, Sun YT, Thoma NH et al. (2002). BRCA2 function in DNA binding and recombination from a BRCA2–DSS1–ssDNA structure. Science 297: 1837–1848.
Yoshida K, Kondoh G, Matsuda Y, Habu T, Nishimune Y, Morita T . (1998). The mouse recA-like gene DMC1 is required for homologous chromosome synapsis during meiosis. Mol Cell 1: 707–718.
Yu VPCC, Köehler M, Steinlein C, Schmid M, Hanakahi LA, van Gool AJ et al. (2000). Gross chromosomal rearrangements and genetic exchange between non-homologous chromosomes following BRCA2 inactivation. Genes Dev 14: 1400–1406.
Yuan S-SF, Lee S-Y, Chen G, Song M, Tomlinson GE, Lee EY . (1999). BRCA2 is required for ionizing radiation-induced assembly of RAD51 complex in vivo. Cancer Res 59: 3547–3551.
Acknowledgements
We thank Dr Uli Rass for critical reading of the manuscript. This work was supported by Cancer Research UK, the EU DNA Repair Consortium, the Breast Cancer Campaign, and the Louis-Jeantet Foundation. TT is a recipient of a post-doctoral fellowship from the Carlsberg foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thorslund, T., West, S. BRCA2: a universal recombinase regulator. Oncogene 26, 7720–7730 (2007). https://doi.org/10.1038/sj.onc.1210870
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210870
Keywords
This article is cited by
-
Progress and prospects in research and clinical practice of hormone receptor-positive, HER-2-negative breast cancer with BRCA1/2 mutations
Discover Oncology (2023)
-
1H, 13C and 15N backbone resonance assignment of the human BRCA2 N-terminal region
Biomolecular NMR Assignments (2020)
-
Unique molecular mechanisms for maintenance and alteration of genetic information in the budding yeast Saccharomyces cerevisiae
Genes and Environment (2017)
-
Loss of BRCA1 or BRCA2 markedly increases the rate of base substitution mutagenesis and has distinct effects on genomic deletions
Oncogene (2017)
-
Investigating BRCA Mutations: A Breakthrough in Precision Medicine of Castration-Resistant Prostate Cancer
Targeted Oncology (2016)