Abstract
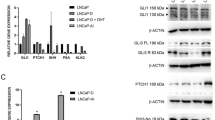
The growth and progression of prostate cancer are dependent on androgens and androgen receptor (AR), which act by modulating gene expression. Utilizing a gene microarray approach, we have identified the α1-subunit gene of soluble guanylyl cyclase (sGC) as a novel androgen-regulated gene. A heterodimeric cytoplasmic protein composed of one α and one β subunit, sGC mediates the widespread cellular effects of nitric oxide (NO). We report here that, in prostate cancer cells, androgens stimulate the expression of sGCα1. A cloned human sGCα1 promoter is activated by androgen in an AR-dependent manner, suggesting that sGCα1 may be a direct AR target gene. Disruption of sGCα1 expression severely compromises the growth of both androgen-dependent and androgen-independent AR-positive prostate cancer cells. Overexpression of sGCα1 alone is sufficient for stimulating prostate cancer cell proliferation. Interestingly, the major growth effect of sGCα1 is independent of NO and cyclic guanosine monophosphate, a major mediator of the sGC enzyme. These data strongly suggest that sGCα1 acts in prostate cancer via a novel pathway that does not depend on sGCβ1. Tissue studies show that sGCα1 expression is significantly elevated in advanced prostate cancer. Thus, sGCα1 may be an important mediator of the procarcinogenic effects of androgens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Arnold JT, Isaacs JT . (2002). Mechanisms involved in the progression of androgen-independent prostate cancers: it is not only the cancer cell's fault. Endocr-Relat Cancer 9: 61–73.
Chang C, Kokontis J, Liao S . (1988). Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science 240: 324–326.
Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R et al. (2004). Molecular determinants of resistance to antiandrogen therapy. Nat Med 10: 33–39.
Chen ME, Lin SH, Chung LW, Sikes RA . (1998). Isolation and characterization of Page-1 and Gage-7 new genes expressed in the LNCaP prostate cancer progression model that share homology with melanoma-associated antigens. J Biol Chem 273: 17618–17625.
Chen S-Y, Cai C, Fisher CJ, Zheng Z, Omwancha J, Hsieh C-L et al. (2006). c-Jun enhancement of androgen receptor transactivation is associated with prostate cancer cell proliferation. Oncogene (in press) [Epub May 29].
Chen Z-J, Vetter M, Che D, Liu S, Tsai M-L, Chang C-H . (2002). The bradykinin/soluble guanylate cyclase signaling pathway is impaired in androgen-independent prostate cancer cells. Cancer Lett 177: 181–187.
Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ et al. (1987). The endocrinology and developmental biology of the prostate. Endocr Rev 8: 338–362.
Eder IE, Hoffmann J, Rogatsch H, Schafer G, Zopf D, Bartsch G et al. (2002). Inhibition of LNCaP prostate tumor growth in vivo by an antisense oligonucleotide directed against the human androgen receptor. Cancer Gene Ther 9: 117–125.
Garcia-Arenas R, Lin FF, Lin D, Jin LP, Shih CC, Chang C et al. (1995). The expression of prostatic acid phosphatase is transcriptionally regulated in human prostate carcinoma cells. Mol Cell Endocrinol 111: 29–37.
Han G, Buchanan G, Ittmann M, Harris JM, Yu X, DeMayo FJ et al. (2005). Mutation of the androgen receptor causes oncogenic transformation of the prostate. Proc Natl Acad Sci USA 102: 1151–1156.
Heisler LE, Evangelou A, Lew AM, Trachtenberg J, Elsholtz HP, Brown TJ . (1997). Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol Cell Endocrin 126: 59–73.
Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM et al. (1983). LNCaP model of human prostatic carcinoma. Cancer Res 43: 1809–1818.
Igawa T, Lin FF, Lee MS, Karan D, Batra SK, Lin MF . (2002). Establishment and characterization of androgen-independent human prostate cancer LNCaP cell model. Prostate 50: 222–235.
Isaacs JT . (1984). Antagonistic effect of androgen on prostatic cell death. Prostate 5: 545–557.
Isaacs JT . (1994). Role of androgens in prostatic cancer. Vitam Horm 49: 433–502.
Isaacs JT . (1999). The biology of hormone refractory prostate cancer. Why does it develop? Urol Clin N Am 26: 263–273.
Jenster G . (1999). The role of the androgen receptor in the development and progression of prostate cancer. Semin Oncol 26: 407–421.
Jia L, Kim J, Shen H, Clark PE, Tilley WD, Coetzee GA . (2003). Androgen receptor activity at the prostate specific antigen locus: steroidal and nonsteroidal mechanisms. Mol Cancer Res 1: 385–392.
Jiang J, Wang LF, Fang YH, Jin FS, Jin WS . (2004). Proliferative response of human prostate cancer cell to hormone inhibited by androgen receptor antisense RNA. Chin Med J (Engl) 117: 684–688.
Kastner P, Mark M, Chambon P . (1995). Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell 83: 859–869.
Koksal IT, Ozcan F, Kilicaslan I, Tefekli A . (2002). Expression of E-cadherin in prostate cancer in formalin-fixed, paraffin-embedded tissues: correlation with pathological features. Pathology 34: 223–238.
Krumenacker JS, Hanafy KA, Murad F . (2004). Regulation of nitric oxide and soluble guanylyl cyclase. Brain Res Bull 62: 505–515.
Krumenacker JS, Hyder SM, Murad F . (2001). Estradiol rapidly inhibits soluble guanylyl cyclase expression in rat uterus. Proc Natl Acad Sci USA 23: 170–178.
Lee C, Sutkowski DM, Sensibar JA, Zelner D, Kim I, Amsel I et al. (1995). Regulation of proliferation and production of prostate-specific antigen in androgen-sensitive prostatic cancer cells, LNCaP, by dihydrotestosterone. Endocrine 136: 796–803.
Lin MF, Meng TC, Rao PS, Chang C, Schonthal AH, Lin FF . (1998). Expression of human prostatic acid phosphatase correlates with androgen-stimulated cell proliferation in prostate cancer cell lines. J Biol Chem 273: 5939–5947.
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K et al. (1995). The nuclear receptor superfamily: the second decade. Cell 83: 835–839.
Meehan KL, Sadar MD . (2003). Androgens and androgen receptor in prostate and ovarian malignancies. Front Biosci 8: 780–800.
Mimeault M, Jouy N, Depreux P, Henichart JP . (2005). Synergistic antiproliferative and apoptotic effects induced by mixed epidermal growth factor receptor inhibitor ZD1839 and nitric oxide donor in human prostatic cancer cell lines. Prostate 62: 187–199.
Papandreou CN, Usmani B, Geng Y, Bogenrieder T, Freeman R, Wilk S et al. (1998). Neutral endopeptidase 24.11 loss in metastatic human prostate cancer contributes to androgen-independent progression. Nat Med 4: 50–57.
Postovit LM, Adams MA, Lash GE, Heaton JP, Graham CH . (2002). Oxygen-mediated regulation of tumor cell invasiveness. Involvement of a nitric oxide signaling pathway. J Biol Chem 277: 35370–35377.
Schrammel A, Behrends S, Schmidt K, Koesling D, Mayer B . (1996). Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol Pharmacol 50: 1–5.
Shenk JL, Fisher CJ, Chen SY, Zhou XF, Tillman K, Shemshedini L . (2001). p53 represses androgen-induced transactivation of prostate-specific antigen by disrupting hAR amino- to carboxyl-terminal interaction. J Biol Chem 276: 38472–38479.
Sinnaeve P, Chiche JD, Nong Z, Varenne O, Van Pelt N, Gillijns H et al. (2001). Soluble guanylate cyclase alpha(1) and beta(1) gene transfer increases NO responsiveness and reduces neointima formation after balloon injury in rats via antiproliferative and antimigratory effects. Circ Res 88: 103–109.
Trapman J, Cleutjens KB . (1997). Androgen-regulated gene expression in prostate cancer. Semin Cancer Biol 8: 29–36.
Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG et al. (2002). The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419: 624–629.
Young CYF, Murtha PE, Zhang J . (1994). Tumor-promoting phorbol ester-induced cell death and gene expression in a human prostate adenocarcinoma cell line. Oncol Res 6: 203–210.
Yuan S, Trachtenberg J, Mills GB, Brown TJ, Xu F, Keating A . (1993). Androgen-induced inhibition of cell proliferation in an androgen-insensitive prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. Cancer Res 53: 1304–1311.
Acknowledgements
We thank Drs P Chambon, H Gronemeyer and S Leisner for critical reading of this paper, Dr X Yuan for providing the AR siRNA oligonucleotides, and Dr D Moorhead and Mei Yang for help with statistical analysis of data. This work was supported by grants from National Institutes of Health and Ohio Cancer Research Associates.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Rights and permissions
About this article
Cite this article
Cai, C., Chen, SY., Zheng, Z. et al. Androgen regulation of soluble guanylyl cyclaseα1 mediates prostate cancer cell proliferation. Oncogene 26, 1606–1615 (2007). https://doi.org/10.1038/sj.onc.1209956
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209956
Keywords
This article is cited by
-
TMPRSS2-ERG activates NO-cGMP signaling in prostate cancer cells
Oncogene (2019)
-
Soluble guanylyl cyclase α1 subunit is a key mediator of proliferation, survival, and migration in ECC-1 and HeLa cell lines
Scientific Reports (2019)
-
cGMP‐independent anti‐tumour actions of the inhibitor of soluble guanylyl cyclase, ODQ, in prostate cancer cell lines
British Journal of Pharmacology (2008)