Abstract
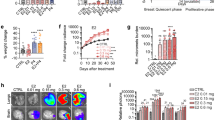
Epidemiological studies indicate that parity enhances HER2/ErbB2/Neu-induced breast tumorigenesis. Furthermore, recent studies using multiparous, ErbB2/Neu-overexpressing mouse mammary tumor virus (MMTV-Neu) mice have shown that parity induces a population of cells that are targeted for ErbB2/Neu-induced transformation. Although parity accelerates mammary tumorigenesis, the pattern of tumor development in multiparous MMTV-Neu mice remains stochastic, suggesting that additional events are required for ErbB2/Neu to cause mammary tumors. Whether such events are genetic in nature or reflective of the dynamic hormonal control of the gland that occurs with pregnancy remains unclear. We postulated that young age at pregnancy initiation or chronic trophic maintenance of mammary epithelial cells might provide a cellular environment that significantly increases susceptibility to ErbB2/Neu-induced tumorigenesis. MMTV-Neu mice that were maintained pregnant or lactating beginning at 3 weeks of age demonstrated accelerated tumorigenesis, but this process was still stochastic, indicating that early pregnancy does not provide the requisite events of tumorigenesis. However, bitransgenic mice that were generated by breeding MMTV-Neu mice with a luteinizing hormone-overexpressing mouse model of ovarian hyperstimulation developed multifocal mammary tumors in an accelerated, synchronous manner compared to virgin MMTV-Neu animals. This synchrony of tumor development in the bitransgenic mice suggests that trophic maintenance of the mammary gland provides the additional events required for tumor formation and maintains the population of cells that are targeted by ErbB2/Neu for transformation. Both the synchrony of tumor appearance and the ability to characterize a window of commitment by ovariectomy/palpation studies permitted microarray analysis to evaluate changes in gene expression over a defined timeline that spans the progression from normal to preneoplastic mammary tissue. These approaches led to identification of several candidate genes whose expression changes in the mammary gland with commitment to ErbB2/Neu-induced tumorigenesis, suggesting that they may either be regulated by ErbB2/Neu and/or contribute to tumor formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
Accessions
GenBank/EMBL/DDBJ
References
Andrechek ER, Hardy WR, Laing MA, Muller WJ . (2004). Proc Natl Acad Sci USA 101: 4984–4989.
Anisimov VN, Popovich IG, Alimova IN, Zabezhinski MA, Semenchenko AV, Yashin AI . (2003). Cancer Lett 193: 49–55.
Beckmann MW, Niederacher D, Schnurch HG, Gusterson BA, Bender HG . (1997). J Mol Med 75: 429–439.
Board M, Humm S, Newsholme EA . (1990). Biochem J 265: 503–509.
Boutros R, Fanayan S, Shehata M, Byrne JA . (2004). Biochem Biophys Res Commun 325: 1115–1121.
Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C et al. (2001). Nat Genet 29: 365–371.
Calin GA, di iasio MG, Caprini E, Vorechovsky I, Natali PG, Sozzi G et al. (2000). Oncogene 19: 1191–1195.
Cao Y, Dave KB, Doan TP, Prescott SM . (2001). Cancer Res 61: 8429–8434.
Cao Y, Pearman AT, Zimmerman GA, McIntyre TM, Prescott SM . (2000). Proc Natl Acad Sci USA 97: 11280–11285.
Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ et al. (2000). Oncogene 19: 968–988.
Cardiff RD, Wellings SR . (1999). J Mammary Gland Biol Neoplasia 4: 105–122.
Dutu R, Nedelea M, Veluda G, Burculet V . (1980). Acta Cytol 24: 160–166.
Gebre-Medhin M, Kindblom LG, Wennbo H, Tornell J, Meis-Kindblom JM . (2001). Am J Pathol 158: 1217–1222.
Gunzburg WH, Salmons B . (1992). Biochem J 283 (Part 3): 625–632.
Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ . (1992). Proc Natl Acad Sci USA 89: 10578–10582.
Hanahan D, Weinberg RA . (2000). Cell 100: 57–70.
Hancock SL, Tucker MA, Hoppe RT . (1993). J Natl Cancer Inst 85: 25–31.
Hennighausen L, Robinson GW . (2001). Dev Cell 1: 467–475.
Hennipman A, Smits J, van Oirschot B, van Houwelingen JC, Rijksen G, Neyt JP et al. (1987). Tumour Biol 8: 251–263.
Henry MD, Triplett AA, Oh KB, Smith GH, Wagner KU . (2004). Oncogene 23: 6980–6985.
Kelsey JL, Gammon MD, John EM . (1993). Epidemiol Rev 15: 36–47.
Kero J, Poutanen M, Zhang FP, Rahman N, McNicol AM, Nilson JH et al. (2000). J Clin Invest 105: 633–641.
Kurokawa Y, Matoba R, Nakamori S, Takemasa I, Nagano H, Dono K et al. (2004). J Exp Clin Cancer Res 23: 135–141.
Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO . (1994). N Engl J Med 331: 5–9.
Land CE, Tokunaga M, Koyama K, Soda M, Preston DL, Nishimori I et al. (2003). Radiat Res 160: 707–717.
Landis MD, Seachrist DD, Montanez-Wiscovich ME, Danielpour D, Keri RA . (2005). Oncogene 24: 5173–5190.
Liang YC, Wu CH, Chu JS, Wang CK, Hung LF, Wang YJ et al. (2005). World J Gastroenterol 11: 2557–2563.
Liu Q, Wuu J, Lambe M, Hsieh SF, Ekbom A, Hsieh CC . (2002). Cancer Causes Control 13: 299–305.
Macheda ML, Rogers S, Best JD . (2005). J Cell Physiol 202: 654–662.
Mazurek S, Grimm H, Boschek CB, Vaupel P, Eigenbrodt E . (2002). Br J Nutr 87 (Suppl 1): S23–S29.
McDermott EW, Barron ET, Smyth PP, O'Higgins NJ . (1990). Br J Surg 77: 1179–1182.
Milliken EL, Ameduri RK, Landis MD, Behrooz A, Abdul-Karim FW, Keri RA . (2002). Endocrinology 143: 3671–3680.
Reed W, Sandstad B, Holm R, Nesland JM . (2003). Int J Surg Pathol 11: 65–74.
Risma KA, Clay CM, Nett TM, Wagner T, Yun J, Nilson JH . (1995). Proc Natl Acad Sci USA 92: 1322–1326.
Robertson C, Primic-Zakelj M, Boyle P, Hsieh CC . (1997). Int J Cancer 73: 1–9.
Russo IH, Russo J . (1998). J Mammary Gland Biol Neoplasia 3: 49–61.
Russo J, Russo IH . (1996). Breast Cancer Res Treat 39: 7–20.
Siegel PM, Dankort DL, Hardy WR, Muller WJ . (1994). Mol Cell Biol 14: 7068–7077.
Singletary KW, McNary MQ, Odoms AM, Nelshoppen J, Wallig MA . (1991). Nutr Cancer 16: 13–23.
Stighall M, Manetopoulos C, Axelson H, Landberg G . (2005). Int J Cancer 115: 403–411.
Sung YK, Hwang SY, Park MK, Bae HI, Kim WH, Kim JC et al. (2003). Cancer Sci 94: 421–424.
Tokunaga M, Norman Jr JE, Asano M, Tokuoka S, Ezaki H, Nishimori I et al. (1979). J Natl Cancer Inst 62: 1347–1359.
Treurniet HF, Rookus MA, Peterse HL, Hart AA, van Leeuwen FE . (1992). Cancer Res 52: 2344–2345.
Warburg O . (1956). Science 123: 309–314.
Wu K, Zhang Y, Xu XC, Hill J, Celestino J, Kim HT et al. (2002). Cancer Res 62: 6376–6380.
Yang X, Edgerton SM, Kosanke SD, Mason TL, Alvarez KM, Liu N et al. (2003). Cancer Res 63: 2425–2433.
Acknowledgements
We extend our gratitude to John Nilson for providing the LH-overexpressing mice and to Kristen Lozada for her dedicated technical support. Histology and microarray hybridization were provided by the core facilities of the CASE Comprehensive Cancer Center (P30-CA43703). This work was supported by a National Institutes of Health Grant (RO1-CA90398, RAK), a USAMRMC Breast Cancer Research Program Predoctoral Traineeship Award (DAMD17-03-1-0302, MDL), and the National Institutes of Health Molecular Therapeutics Training Program (GM08803, MDL).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Landis, M., Seachrist, D., Abdul-Karim, F. et al. Sustained trophism of the mammary gland is sufficient to accelerate and synchronize development of ErbB2/Neu-induced tumors. Oncogene 25, 3325–3334 (2006). https://doi.org/10.1038/sj.onc.1209365
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209365
Keywords
This article is cited by
-
Loss of RPTPγ primes breast tissue for acid extrusion, promotes malignant transformation and results in early tumour recurrence and shortened survival
British Journal of Cancer (2022)
-
Na+,HCO3–-cotransporter NBCn1 (Slc4a7) accelerates ErbB2-induced breast cancer development and tumor growth in mice
Oncogene (2018)
-
Disrupting Na+,HCO3–-cotransporter NBCn1 (Slc4a7) delays murine breast cancer development
Oncogene (2016)
-
Interleukin-33 in tumorigenesis, tumor immune evasion, and cancer immunotherapy
Journal of Molecular Medicine (2016)
-
A new role of SNAI2 in postlactational involution of the mammary gland links it to luminal breast cancer development
Oncogene (2015)