Abstract
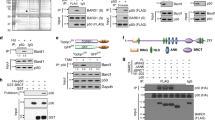
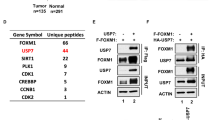
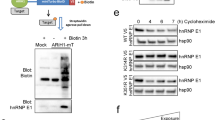
SIAH-1 and SIAH-2 are the human members of an evolutionary highly conserved E3 ligase family. SIAH-1 is a p53 and p21Waf−1/Cip−1 induced gene during apoptosis and tumor suppression. In stable-transfected clones of MCF-7 cells, SIAH-1 overexpression was associated with apoptosis, mitotic alterations and p21Waf−1/Cip−1 induction of expression. Using a two-hybrid screening, we identified here the transcriptional corepressor CtBP-interacting protein (CtIP) as a SIAH-1-interacting protein. CtIP has been proposed as a regulator of p21Waf−1/Cip−1 gene transcription through a protein complex involving BRCA1. We demonstrate that SIAH-1 associates with CtIP both in vitro and in vivo. This interaction led to CtIP degradation by the ubiquitin–proteasome pathway. As expected, SIAH-1 induced p21Waf−1/Cip−1 transcription in Jurkat-T cell. Surprisingly, a SIAH protein deleted of its RING finger, SIAH-1ΔN, which is able to interact with CtIP but does not promote its degradation, also induced transcription from the p21Waf−1 promoter in a similar extent as did SIAH-1. Our results suggest that p21Waf−1/Cip−1 induction by SIAH-1 could not be mediated by CtIP degradation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Antonio C, Ferby I, Wilhelm H, Jones M, Karsenti E, Nebreda AR and Vernos I . (2000). Cell, 102, 425–435.
Boehm J, He Y, Greiner A, Staudt L and Wirth T . (2001). EMBO J., 20, 4153–4162.
Bruzzoni-Giovanelli H, Faille A, Linares-Cruz G, Nemani M, Le Deist F, Germani A, Chassoux D, Millot G, Roperch JP, Amson R, Telerman A and Calvo F . (1999). Oncogene, 18, 7101–7109.
Chen Y, Lee WH and Chew HK . (1999). J. Cell. Physiol., 181, 385–392.
Funabiki H and Murray AW . (2000). Cell, 102, 411–424.
Germani A, Bruzzoni-Giovanelli H, Fellous A, Gisselbrecht S, Varin-Blank N and Calvo F . (2000). Oncogene, 19, 5997–6006.
Germani A, Romero F, Houlard M, Camonis J, Gisselbrecht S, Fischer S and Varin-Blank N . (1999). Mol. Cell. Biol., 19, 3798–3807.
Koipally J and Georgopoulos K . (2002). J. Biol. Chem., 277, 23143–23149.
Li S, Chen PL, Subramanian T, Chinnadurai G, Tomlinson G, Osborne CK, Sharp ZD and Lee WH . (1999). J. Biol. Chem., 274, 11334–11338.
Li S, Li Y, Carthew RW and Lai ZC . (1997). Cell, 90, 469–478.
Li S, Ting NS, Zheng L, Chen PL, Ziv Y, Shiloh Y, Lee EY and Lee WH . (2000). Nature, 406, 210–215.
Linares-Cruz G, Bruzzoni-Giovanelli H, Alvaro V, Roperch JP, Tuynder M, Schoevaert D, Nemani M, Prieur S, Lethrosne F, Piouffre L, Reclar V, Faille A, Chassoux D, Dausset J, Amson RB, Calvo F and Telerman A . (1998). Proc. Natl. Acad. Sci. USA, 95, 1131–1135.
Liu J, Stevens J, Rote CA, Yost HJ, Hu Y, Neufeld KL, White RL and Matsunami N . (2001). Mol. Cell, 7, 927–936.
Matsuzawa S, Li C, Ni CZ, Takayama S, Reed JC and Ely KR . (2003). J. Biol. Chem., 278, 1837–1840.
Matsuzawa SI and Reed JC . (2001). Mol. Cell, 7, 915–926.
Meloni AR, Smith EJ and Nevins JR . (1999). Proc. Natl. Acad. Sci. USA, 96, 9574–9579.
Nemani M, Linares-Cruz G, Bruzzoni-Giovanelli H, Roperch JP, Tuynder M, Bougueleret L, Cherif D, Medhioub M, Pasturaud P, Alvaro V, der Sarkissan H, Cazes L, Le Paslier D, Le Gall I, Israeli D, Dausset J, Sigaux F, Chumakov I, Oren M, Calvo F, Amson RB, Cohen D and Telerman A . (1996). Proc. Natl. Acad. Sci. USA, 93, 9039–9042.
Polekhina G, House CM, Traficante N, Mackay JP, Relaix F, Sassoon DA, Parker MW and Bowtell DD . (2002). Nat. Struct. Biol., 9, 68–75.
Schaeper U, Subramanian T, Lim L, Boyd JM and Chinnadurai G . (1998). J. Biol. Chem., 273, 8549–8552.
Tang AH, Neufeld TP, Kwan E and Rubin GM . (1997). Cell, 90, 459–467.
Tiedt R, Bartholdy BA, Matthias G, Newell JW and Matthias P . (2001). EMBO J., 20, 4143–4152.
Wheeler TC, Chin LS, Li Y, Roudabush FL and Li L . (2002). J. Biol. Chem., 277, 10273–10282.
Wong AK, Ormonde PA, Pero R, Chen Y, Lian L, Salada G, Berry S, Lawrence Q, Dayananth P, Ha P, Tavtigian SV, Teng DH and Bartel PL . (1998). Oncogene, 17, 2279–2285.
Wu-Baer F and Baer R . (2001). Nature, 414, 36.
Yu X, Wu LC, Bowcock AM, Aronheim A and Baer R . (1998). J. Biol. Chem., 273, 25388–25392.
Zhang J, Guenther MG, Carthew RW and Lazar MA . (1998). Genes Dev., 12, 1775–1780.
Acknowledgements
We thank Dr R Baer for the kind gift of CtIP plasmid and anti-CtIP antibodies, Dr. Feunteun for anti-BRCA1 antibodies and Dr Fusco for HA-CtIP plasmid. This work was partially supported by grants from Eli Lilly International Foundation, the ‘ECOS-Sud’ program (action U97S03), the ‘Association pour la Recherche Contre le Cancer (ARC)’ and ‘La Ligue Contre le Cancer’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Germani, A., Prabel, A., Mourah, S. et al. SIAH-1 interacts with CtIP and promotes its degradation by the proteasome pathway. Oncogene 22, 8845–8851 (2003). https://doi.org/10.1038/sj.onc.1206994
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1206994
Keywords
This article is cited by
-
Growth factor TGF-β induces intestinal epithelial cell (IEC-6) differentiation: miR-146b as a regulatory component in the negative feedback loop
Genes & Nutrition (2013)
-
Pathway choice in DNA double strand break repair: observations of a balancing act
Genome Integrity (2012)
-
Mre11 regulates CtIP-dependent double-strand break repair by interaction with CDK2
Nature Structural & Molecular Biology (2012)
-
Siah1 proteins enhance radiosensitivity of human breast cancer cells
BMC Cancer (2010)
-
Distinct expression patterns of the E3 ligase SIAH-1 and its partner Kid/KIF22 in normal tissues and in the breast tumoral processes
Journal of Experimental & Clinical Cancer Research (2010)