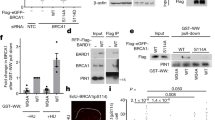
Abstract
Mutations in the BRCA1 and BRCA2 genes predispose women to familial, early-onset breast cancer. Both the BRCA1 and BRCA2 proteins appear to function in the homologous recombination pathway of DNA double-strand break repair. Both BRCA1 and BRCA2 have also been implicated in transcription by RNA polymerase II, for both proteins have domains which, when tethered adjacent to a promoter, can activate transcription. In experiments reported here, we have used protein affinity chromatography and coimmunoprecipitation techniques to show that the putative N-terminal acidic transcriptional activation domain of BRCA2 interacts with replication protein A (RPA), a protein essential for DNA repair, replication and recombination. This interaction was not mediated by DNA and was specific for human RPA but not yeast RPA. Since the cancer-predisposing mutation Y42C in BRCA2 significantly compromised the interaction between RPA and BRCA2, this interaction may be biologically important. That BRCA2 protein in HeLa cell extract also coimmunoprecipitated with RPA suggested that this interaction occurs in vivo. Therefore, the transcriptional activation domains within BRCA2, and perhaps BRCA1, may provide links to RPA and DNA repair processes rather than transcription.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bardwell AJ, Bardwell L, Iyer N, Svejstrup JQ, Feaver WJ, Kornberg RD and Friedberg EC . (1994). Mol. Cell Bio., 14, 3569–3576.
Chapman MS and Verma IM . (1996). Nature, 382, 678–679.
Chen J, Silver DP, Walpita D, Cantor SB, Gazdar AF, Tomlinson G, Couch FJ, Weber BL, Ashley T, Livingston DM and Scully R . (1998). Mol. Cell, 2, 317–328.
Chen PL, Chen CF, Chen Y, Xiao J, Sharp ZD and Lee WH . (1998). Proc. Natl. Acad. Sci. USA, 95, 5287–5292.
Choudhary SK and Li R . (2002). J. Cell Biochem., 84, 666–674.
Connor F, Bertwistle D, Mee PJ, Ross GM, Swift S, Grigorieva E, Tybulewicz VL and Ashworth A . (1997). Nat. Genet., 17, 423–430.
Davies AA, Masson J-Y, McIlwraith MJ, Stasiak AZ, Stasiak A, Venkitaraman AR and West SC . (2001). Mol. Cell, 7, 273–282.
de Laat WL, Appeldoorn E, Sugasawa K, Weterings E, Jaspers NG and Hoeijmakers JH . (1998). Genes Dev., 12, 2598–2609.
Dutta A, Ruppert JM, Aster JC and Winchester E . (1993). Nature, 365, 79–82.
Golub EI, Gupta RC, Haaf T, Wold MS and Radding CM . (1998). Nucleic Acids Res., 6, 5388–5393.
Greenblatt J and Ingles CJ . (1996). Methods Enzymol., 274, 120–133.
He Z, Brinton BT, Greenblatt J, Hassell JA and Ingles CJ . (1993). Cell, 73, 1223–1232.
He Z, Henricksen LA, Wold MS and Ingles CJ . (1995). Nature, 374, 566–569.
He Z, Wong JM, Maniar HS, Brill SJ and Ingles CJ . (1996). J. Biol. Chem., 271, 28243–28249.
Hu YF, Hao ZL and Li R . (1999). Genes Dev., 13, 637–642.
Kerr P and Ashworth A . (2001). Curr. Biol., 11, R668–R676.
Li R and Botchan MR . (1993). Cell, 73, 1207–1221.
Lim DS and Hasty P . (1996). Mol. Cell Biol., 16, 7133–7143.
Ma J and Ptashne M . (1987). Cell, 9, 113–119.
Marmorstein LY, Ouchi T and Aaronson SA . (1998). Proc. Natl. Acad. Sci. USA, 95, 13869–13874.
McIlwraith MJ, Van Dyck E, Masson JY, Stasiak AZ, Stasiak A and West SC . (2000). J. Mol. Biol., 304, 151–164.
Milner J, Ponder B, Hughes-Davies L, Seltmann M and Kouzarides T . (1997). Nature, 386, 772–723.
Mizuta R, LaSalle JM, Cheng HL, Shinohara A, Ogawa H, Copeland N, Jenkins NA, Lalande M and Alt FW . (1997). Proc. Natl. Acad. Sci. USA, 94, 6927–6932.
Monteiro AN, August A and Hanafusa H . (1996). Proc. Natl. Acad. Sci. USA, 93, 13595–13599.
New JH, Sugiyama T, Zaitseva E and Kowalczykowski SC . (1998). Nature, 391, 407–410.
Nordling M, Karlsson P, Wahlstrom J, Engwall Y, Wallgren A and Martinsson T . (1998). Cancer Res., 58, 1372–1375.
Pao GM, Janknecht R, Ruffner H, Hunter T and Verma IM . (2000). Proc. Natl. Acad. Sci. USA, 97, 1020–1025.
Patel KJ, Vu VP, Lee H, Corcoran A, Thistlethwaite FC, Evans MJ, Colledge WH, Friedman LS, Ponder BA and Venkitaraman AR . (1998). Mol. Cell, 1, 347–357.
Rahman N and Stratton MR . (1998). Annu. Rev. Genet., 32, 95–121.
Scully R, Anderson SF, Chao DM, Wei W, Ye L, Young RA, Livingston DM and Parvin JD . (1997) Proc. Natl. Acad. Sci. USA, 94, 5605–5610.
Sharan SK, Morimatsu M, Albrecht U, Lim DS, Regel E, Dinh C, Sands A, Eichele G, Hasty P and Bradley A . (1997). Nature, 386, 804–810.
Shinohara A, Shinohara M, Ohta T, Matsuda S and Ogawa T . (1998). Genes Cells, 3, 145–156.
Sonoda E, Sasaki MS, Buerstedde JM, Bezzubova O, Shinohara A, Ogawa H, Takata M, Yamaguchi-Iwai Y and Takeda S . (1998). EMBO J., 17, 598–608.
Sopta M, Carthew RW and Greenblatt J . (1985). J. Biol. Chem., 260, 10353–10360.
van Golen K, Milliron K, Davies S and Merajver SD . (1999). J. Lab. Clin. Med., 134, 11–18.
Venkitaraman AR . (2002) Cell, 108, 171–182.
Warren M, Smith A, Partridge N, Masabanda J, Griffin D and Ashworth A . (2002). Hum. Mol. Genet., 11, 841–851.
Wong AKC, Pero R, Ormonde PA, Tavtigian SV and Bartel PL . (1997). J. Biol. Chem., 272, 31941–31944.
Ye Q, Hu YF, Zhong H, Nye AC, Belmont AS and Li R . (2001). J. Cell Biol., 155, 911–921.
Acknowledgements
JMSW (deceased) and DI made equal contributions. We thank Michael Shales for assistance with preparation of the manuscript. This work was supported by a grant to CJI from the National Cancer Institute of Canada with funds from the Canadian Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wong, J., Ionescu, D. & Ingles, C. Interaction between BRCA2 and replication protein A is compromised by a cancer-predisposing mutation in BRCA2. Oncogene 22, 28–33 (2003). https://doi.org/10.1038/sj.onc.1206071
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1206071
Keywords
This article is cited by
-
Elevated Expression of RPA3 Is Involved in Gastric Cancer Tumorigenesis and Associated with Poor Patient Survival
Digestive Diseases and Sciences (2017)
-
RPA-coated single-stranded DNA as a platform for post-translational modifications in the DNA damage response
Cell Research (2015)
-
Human single-stranded DNA binding proteins are essential for maintaining genomic stability
BMC Molecular Biology (2013)
-
Structure‐based protein‐protein interaction networks and drug design
Quantitative Biology (2013)
-
Theoretical prediction of the binding free energy for mutants of replication protein A
Journal of Molecular Modeling (2012)