Abstract
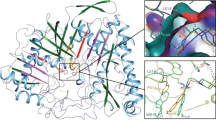
Mammalian carboxylesterases cleave the anticancer prodrug CPT-11 (Irinotecan) into SN-38, a potent topoisomerase I poison, and 4-piperidino-piperidine (4PP). We present the 2.5 Å crystal structure of rabbit liver carboxylesterase (rCE), the most efficient enzyme known to activate CPT-11 in this manner, in complex with the leaving group 4PP. 4PP is observed bound adjacent to a high-mannose Asn-linked glycosylation site on the surface of rCE. This product-binding site is separated from the catalytic gorge by a thin wall of amino acid side chains, suggesting that 4PP may be released through this secondary product exit pore. The crystallographic observation of a leaving group bound on the surface of rCE supports the 'back door' product exit site proposed for the acetylcholinesterases. These results may facilitate the design of improved anticancer drugs or enzymes for use in viral-directed cancer cotherapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Williams, F.M. Clin. Pharmacokinet. 10, 392–403 (1985).
Bodor, N. & Buchwald, P. Med. Res. Rev. 20, 58–101 (2000).
Joly, J.M. & Brown, T.M. Toxicol. Appl. Pharmacol. 84, 523–532 (1986).
Brzezinski, M.R. et al. Drug Metab. Dispos. 25, 1089–1096 (1997).
Kamendulis, L.M., Brzezinski, M.R., Pindel, E.V., Bosron, W.F. & Dean, R.A. J. Pharmacol. Exp. Ther. 279, 713–717 (1996).
Lotti, M., Ketterman, A., Waskell, L. & Talcott, R.E. Biochem. Pharmacol. 32, 3735–3738 (1983).
Ollis, D.L. et al. Protein Eng. 5, 197–211 (1992).
Morton, C.L. et al. Cancer Res. 60, 4206–4210 (2000).
Potter, P.M., Pawlik, C.A., Morton, C.L., Naeve, C.W. & Danks, M.K. Cancer Res. 58, 2646–2651 (1998).
Danks, M.K. et al. Clin. Cancer Res. 5, 917–924 (1999).
Khanna, R., Morton, C.L., Danks, M.K. & Potter, P.M. Cancer Res. 60, 4725–4728 (2000).
Pindel, E.V. et al. J. Biol. Chem. 272, 14769–14775 (1997).
Bosron, W.F. & Hurley, T.D. Nature Struct. Biol. 9, 4–5 (2002).
Chabot, G.G. Clin. Pharmacokinet. 33, 245–259 (1997).
Danks, M.K., Morton, C.L., Pawlik, C.A. & Potter, P.M. Cancer Res. 58, 20–22 (1998).
Potter, P.M., Wolverton, J.S., Morton, C.L., Wierdl, M. & Danks, M.K. Cancer Res. 58, 3627–3632 (1998).
Wierdl, M. et al. Cancer Res. 61, 5078–5082 (2001).
Meck, M.M. et al. Cancer Res. 61, 5083–5089 (2001).
Harel, M. et al. Proc. Natl. Acad. Sci. USA 90, 9031–9035 (1993).
Sussman, J.L., Harel, M. & Silman, I. Chem. Biol. Interact. 87, 187–197 (1993).
Kryger, G., Silman, I. & Sussman, J.L. Structure Fold Des. 7, 297–307 (1999).
Schrag, J.D. & Cygler, M. J. Mol. Biol. 230, 575–591 (1993).
Chen, J.C. et al. Biochemistry 37, 5107–5117 (1998).
Helenius, A. & Aebi, M. Science 291, 2364–2369 (2001).
Gilson, M.K. et al. Science 263, 1276–1278 (1994).
Bartolucci, C., Perola, E., Cellai, L., Brufani, M. & Lamba, D. Biochemistry 38, 5714–5719 (1999).
Wallace, T.J., Kodsi, E.M., Langston, T.B., Gergis, M.R. & Grogan, W.M. J. Biol. Chem. 276, 33165–33174 (2001).
Morton, C.L. & Potter, P.M. Mol. Biotechnol. 16, 193–202 (2000).
Otwinowski, Z. & Minor, W. Data collection and processing (Daresbury Laboratories, Warrington; 1993).
Matthews, B.W. J. Mol. Biol. 33, 491–497 (1968).
Navaza, J. & Saludjian, P. Methods Enzymol. 276A, 581–594 (1997).
Brünger, A.T. et al. Acta Crystallogr. D 54, 905–921 (1998).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Acta Crystallogr. A 47, 110–119 (1991).
Read, R.J. Acta Crystallogr. A 42, 140–149 (1986).
Laskowski, R.A., McArthur, M.W., Moss, D.S. & Thornton, J.M. J. Appl. Crystallogr. 26, 283–291 (1993).
Kraulis, P. J. Appl. Crystallogr. 24, 946–950 (1991).
Esnouf, R.M. Acta. Crystallogr. D 55, 938–940 (1999).
Merritt, E.A. & Bacon, D.J. Methods Enzymol. 277, 505–524 (1997).
Holm, L. & Sander, C. Nucleic Acids Res. 25, 231–234 (1997).
Thompson, J.D., Higgins, D.G. & Gibson, T.J. Nucleic Acids Res. 22, 4673–4680 (1994).
Acknowledgements
The authors wish to thank R. Watkins, J. Chrencik, T. Thieu, Y. Xue, E. Collins, L. Betts and the members of the Redinbo Laboratory for discussions and experimental assistance. We also thank G. Pielak, D. Erie and A. Tripathy for assistance with CD thermal denaturation studies. Supported by a Burroughs Wellcome Career Award in the Biomedical Sciences (M.R.R.) and by the NIH and American Lebanese Syrian Associated Charities (P.M.P.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bencharit, S., Morton, C., Howard-Williams, E. et al. Structural insights into CPT-11 activation by mammalian carboxylesterases. Nat Struct Mol Biol 9, 337–342 (2002). https://doi.org/10.1038/nsb790
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb790
This article is cited by
-
Effect of drug metabolizing enzymes and transporters in Thai colorectal cancer patients treated with irinotecan-based chemotherapy
Scientific Reports (2020)
-
The Development of Bacterial Carboxylesterase Biological Recognition Elements for Cocaine Detection
Molecular Biotechnology (2018)
-
Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics
Clinical Pharmacokinetics (2018)
-
Carboxylesterases in lipid metabolism: from mouse to human
Protein & Cell (2018)
-
Simulation on the structure of pig liver esterase
Journal of Molecular Modeling (2011)