Abstract
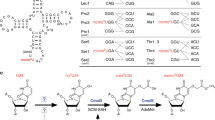
Aminoglycosides bind to RNA and interfere with its function, and it has been suggested that aminoglycoside binding to RNA displaces essential divalent metal ions. Here we demonstrate that addition of various aminoglycosides inhibited Pb2+-induced cleavage of yeast tRNAPhe. Cocrystallization of yeast tRNAPhe and an aminoglycoside, neomycin B, resulted in crystals that diffracted to 2.6 Å and the structure of the complex was solved by molecular replacement. The structure shows that the neomycin B binding site overlaps with known divalent metal ion binding sites in yeast tRNAPhe, providing direct evidence for the hypothesis that aminoglycosides displace metal ions. Additionally, the neomycin B binding site overlaps with major determinants for Escherichia coli phenylalanyl-tRNA-synthetase. Here we present data demonstrating that addition of neomycin B inhibited aminoacylation of E. coli tRNAPhe in the mid μM range. Given that aminoglycoside and metal ion binding sites overlap, we discuss that aminoglycosides can be considered as 'metal mimics'.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Yoshizawa, S., Fourmy, D. & Puglisi, J.D. EMBO J. 17, 6437–6448 (1998).
Stage, T.K., Hertel, K.J. & Uhlenbeck, O.C. RNA 1, 95–101 (1995).
Rogers, J., Chang, A.H., von Ahsen, U., Schroeder, R., & Davies, J. J. Mol. Biol. 259, 916–925 (1996).
von Ahsen, U., Davies, J. & Schroeder, R. Nature 353, 368–370 (1991).
von Ahsen, U., Davies, J. & Schroeder, R. J. Mol. Biol. 226, 935–941 (1992).
Mikkelsen, N.E., Brännvall, M., Virtanen, A., & Kirsebom, L.A. (1999) Proc. Natl. Acad. Sci. USA 96, 6155–6160 (1999).
Hermann, T., & Westhof, E. J. Mol. Biol. 276, 903–912 (1998).
Hoch, I., Berens, C., Westhof, E., & Schroeder, R. J. Mol. Biol. 282, 557–569 (1998).
Feig, A.L. & Uhlenbeck, O.C. In The RNA world: second edition (eds Gestland, R.F., Cech, T.R. & Atkins, J.F.) 287–319 (Cold Spring Harbor Press, Cold Spring Harbor, New York; 1999).
Brown, R.S., Hingerty, B.E., Dewan, J.C. & Klug, A. Nature 303, 543–546 (1983).
Brown, R.S., Dewan, J.C. & Klug, A. Biochemistry 24, 4785–4801 (1985).
Kirk, S.R., & Tor, Y. Bioorg. Med. Chem. 7, 1979–1991 (1999).
Jack, A., Ladner, J.E., Rhodes, D., Brown, R.S. & Klug, A. J. Mol. Biol. 111, 315–328 (1977).
Jovine, L., Djordjevic, S. & Rhodes, D. J. Mol. Biol. 301, 401–414 (2000).
Shi, H. & Moore, P.B. RNA 6, 1091–1105 (2000).
Sussman, J.L., Holbrook, S.R., Warrant, R.W., Church, G.M. & Kim, S.-H. J. Mol. Biol. 123, 607–630 (1978).
Behlen, L.S., Sampson, J.R., DiRenzo, A.B. & Uhlenbeck, O.C. Biochemistry 29, 2515–2523 (1990).
McClain, W.H. & Foss, K. J. Mol. Biol. 202, 697–709 (1988).
Fourmy, D., Recht, M.I., Blanchard, S.C. & Puglisi, J.D. Science 274, 1367–1371 (1996).
Faber, C., Sticht, H., Schweimer, K. & Rösch, P. J. Biol. Chem. 275, 20660–20666 (2000).
Jiang, L., Majumdar, A., Hu, W., Jaishree, T.J. & Patel, D.J. Struct. Fold Des. 7, 817–827 (1999).
Varani, L., Spillantini, M.G., Goedert, M. & Varani, G. Nucleic Acids Res. 28, 710–719 (2000).
Kufel, J. & Kirsebom, L.A. J. Mol. Biol. 263, 685–698 (1996).
Kufel, J. & Kirsebom, L.A. RNA 4, 777–788 (1998).
Otwinowski, Z. In proceedings of the CCP4 study weekend (eds. Sawyer, L., Issacs, N. & Bailey, S.) 56–62 (Daresburry Laboratories, Warrington, UK; 1993).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Acta Crystallogr. A 47, 110–119 (1991).
Kraulis, P. J. Appl. Crystallogr. 24, 946–950 (1991).
Bacon, D.J. & Anderson, W.F. J. Mol. Graph. 6 (1988).
Westhof, E., & Sundaralingam, M. Biochemistry 25, 4868–4878 (1986).
Navaza, J. Acta Crystallogr. A 50, 157–163 (1994).
Brünger, A.T. et al. Acta Crystallogr. D 54, 905–921 (1998).
Acknowledgements
We thank H. Eklund and D. Hughes for discussions and critical reading of the manuscript. We are also grateful to J. Davis for the generous gift of 5-epi-sisomicin. This work was supported by the Strategic Research Foundation and the Swedish Natural Science Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mikkelsen, N., Johansson, K., Virtanen, A. et al. Aminoglycoside binding displaces a divalent metal ion in a tRNA–neomycin B complex. Nat Struct Mol Biol 8, 510–514 (2001). https://doi.org/10.1038/88569
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/88569
This article is cited by
-
Some nontoxic metal-based drugs for selected prevalent tropical pathogenic diseases
JBIC Journal of Biological Inorganic Chemistry (2017)
-
EF4 disengages the peptidyl-tRNA CCA end and facilitates back-translocation on the 70S ribosome
Nature Structural & Molecular Biology (2016)