Abstract
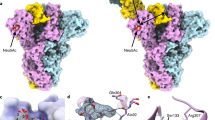
Paramyxoviruses are the main cause of respiratory disease in children. One of two viral surface glycoproteins, the hemagglutinin-neuraminidase (HN), has several functions in addition to being the major surface antigen that induces neutralizing antibodies. Here we present the crystal structures of Newcastle disease virus HN alone and in complex with either an inhibitor or with the β-anomer of sialic acid. The inhibitor complex reveals a typical neuraminidase active site within a β-propeller fold. Comparison of the structures of the two complexes reveal differences in the active site, suggesting that the catalytic site is activated by a conformational switch. This site may provide both sialic acid binding and hydrolysis functions since there is no evidence for a second sialic acid binding site in HN. Evidence for a single site with dual functions is examined and supported by mutagenesis studies. The structure provides the basis for the structure-based design of inhibitors for a range of paramyxovirus-induced diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Choppin, P.W. & Scheid, A. The role of viral glycoproteins in adsorption, penetration and pathogenicity of viruses. Rev. Infect. Dis. 2, 40–61 ( 1980).
Lamb, R.A. & Kolakofsky, D. In Fundamental virology, 3rd Edition. The paramyxoviruses (eds, Fields, B.N. et al.) 577–604 (Lippincott Raven; 1996).
Bousse, T., Takimoto, T., Gorman, W.L., Takahashi, T. & Portner, A. Regions on the hemagglutinin-neuraminidase proteins of human parainfluenza virus type-1 and sendai virus important for membrane fusion. Virology 204, 506– 514 (1994).
Deng, R.T., Wang, Z.Y., Mirza, A.M. & Iorio, R.M. Localization of a domain on the paramyxovirus attachment protein required for the promotion of cellular fusion by its homologous fusion protein spike. Virology 209, 457–469 ( 1995).
Sergel, T., Mcginnes, L.W., Peeples, M.E. & Morrison, T.G. The attachment function of the Newcastle-disease virus hemagglutinin-neuraminidase protein can be separated from fusion promotion by mutation. Virology 193, 717–726 ( 1993).
Tanabayashi, K. & Compans, R.W. Functional interaction of paramyxovirus glycoproteins: identification of a domain in sendai virus HN which promotes cell fusion. J. Virol. 70, 6112–6118 (1996).
Bousse, T., Takimoto, T. & Portner, A. A single amino-acid change enhances the fusion promotion activity of human parainfluenza virus type-1 hemagglutinin-neuraminidase glycoprotein . Virology 209, 654–657 (1995).
Tsurudome, M, et al. Identification of regions on the hemagglutinin-neuraminidase protein of human parainfluenza virus type-2 important for promoting cell fusion . Virology 213, 190–203 (1995).
Watowich, S.J., Skehel, J.J. & Wiley, D.C. Crystal structures of influenza-virus hemagglutinin in complex with high-affinity receptor analogs. Structure 2, 719–731 ( 1994).
Von Itzstein, M. et al. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 363, 418– 423 (1993).
Kim, C.U. et al. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J. Am. Chem. Soc. 119, 681–690 (1997).
Markwell, M.K. & Fox, C.F. Protein-protein interactions within paramyxoviruses identified by native disulfide binding or reversible chemical cross-linking. J. Virol. 33, 152–166 (1980).
Morrison, T.G. Structure, function, and intracellular processing of paramyxovirus membrane proteins. Virus Res. 10, 113– 135 (1988).
Sheehan, J.P., Iorio, R.M., Syddall, R.J., Glickman, R.L. & Bratt, M.A. Reducing agent-sensitive dimerization of the hemagglutinin-neuraminidase glycoprotein of Newcastle-disease virus correlates with the presence of cysteine at residue 123. Virology 161, 603–606 ( 1987).
Mcginnes, L.W. & Morrison, T.G. The role of the individual cysteine residues in the formation of the mature, antigenic HN protein of Newcastle disease virus. Virology 200 , 470–483 (1994).
Takimoto, T., Taylor, G.L., Crennell, S.J., Scroggs, R.A. & Portner, A. Crystallization of Newcastle disease virus hemagglutinin-neuraminidase glycoprotein. Virology 270, 208–214 ( 2000).
Taylor, G. Sialidases: structures, biological significance and therapeutic potential . Curr. Opin. Struct. Biol. 6, 830– 837 (1996).
Colman, P.M., Hoyne, P.A. & Lawrence, M.C. Sequence and structure alignment of paramyxovirus hemagglutinin-neuraminidase with influenza virus neuraminidase. J. Virol. 67, 2972–2980 (1993).
Langedijk, J.P.M., Daus, F.J. & vanoirschot, J.T. Sequence and structure alignment of paramyxoviridae attachment proteins and discovery of enzymatic activity for a morbillivirus hemagglutinin. J. Virol. 71, 6155– 6167 (1997).
Pitt, J.J., dasilva, E. & Gorman, J.J. Determination of the disulfide bond arrangement of Newcastle disease virus hemagglutinin neuraminidase: correlation with a beta-sheet propeller structural fold predicted for paramyxoviridae attachment proteins . J. Biol. Chem. 275, 6469– 6478 (2000).
Mcginnes, L.W. & Morrison, T.G. The role of individual oligosaccharide chains in the activities of the HN glycoprotein of Newcastle-disease virus. Virology 212, 398–410 (1995).
Burmeister, W.P., Henrissat, B., Bosso, C., Cusack, S. & Ruigrok, R.W.H. Influenza-B virus neuraminidase can synthesize its own inhibitor. Structure 1, 19–26 (1993).
Taylor, N.R. & Vonitzstein, M. Molecular modeling studies on ligand-binding to sialidase from influenza-virus and the mechanism of catalysis. J. Med. Chem. 37, 616– 624 (1994).
Barroso, I.M., Moralejo, F.J. & Villar, E. Ionic dependence of the sialidase activity of hemagglutinin-neuraminidase glycoprotein in Newcastle-disease virus membrane . Biochem. Soc. Trans. 22, S366 (1994).
Chong, A.K.J., Pegg, M.S., Taylor, N.R. & Vonitzstein, M. Evidence for a sialosyl cation transition-state complex in the reaction of sialidase from influenza-virus. Eur. J. Biochem. 207 , 335–343 (1992).
Varghese, J.N., McKimmbreschkin, J.L., Caldwell, J.B., Kortt, A.A. & Colman, P.M. The structure of the complex between influenza-virus neuraminidase and sialic-acid, the viral receptor. Proteins 14, 327– 332 (1992).
Varghese, J.N. et al. Structural evidence for a second sialic acid binding site in avian influenza virus neuraminidases. Proc. Natl Acad. Sci. USA 94, 11808–11812 ( 1997).
Mirza, A.M., Deng, R.T. & Iorio, R.M. Site-directed mutagenesis of a conserved hexapeptide in the paramyxovirus hemagglutinin-neuraminidase glycoprotein: effects on antigenic structure and function. J. Virol. 68, 5093–5099 (1994).
Sheehan, J.P. & Iorio, R.M. A single amino-acid substitution in the hemagglutinin-neuraminidase of Newcastle-disease virus results in a protein-deficient in both functions. Virology 189, 778–781 (1992).
Deng, R.T. et al. Mutations in the Newcastle disease virus hemagglutinin-neuraminidase protein that interfere with its ability to interact with the homologous F protein in the promotion of fusion. Virology 253, 43–54 (1999).
Morrison, T.G. & Portner, A. In Paramyxoviruses. Structure, function and intracellular processing of the glycoproteins of paramyxoviridae (ed. Kingsbuy, D.W.) 347–382, (Plenum, New York; 1991).
Scheid, A. & Choppin, P.W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity by proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology 57, 470 –490 (1974).
Merz, D.C., Prehm, P., Scheid, A. & Choppin, P.W. Inhibition of the neuraminidase of paramyxoviruses by halide-ions: a possible means of modulating the two activities of the HN protein. Virology 112, 296–305 (1981).
Thompson, S.D. & Portner, A. Localization of functional sites on the hemagglutinin neuraminidase glycoprotein of sendai virus by sequence-analysis of antigenic and temperature-sensitive mutants . Virology 160, 1–8 (1987).
Weis, W. et al. Structure of the influenza-virus hemagglutinin complexed with its receptor, sialic-acid. Nature 333, 426 –431 (1988).
Yusoff, K., Nesbit, M., Mccartney, H., Emmerson, P.T. & Samson, A.C.R. Mapping of three antigenic sites on the hemagglutinin-neuraminidase protein of Newcastle-disease virus. Virus Res. 11, 319– 333 (1988).
Iorio, R.M., Glickman, R.L., Riel, A.M., Sheehan, J.P. & Bratt, M.A. Functional and neutralization profile of seven overlapping antigenic sites on the HN glycoprotein of Newcastle-disease virus: monoclonal antibodies to some sites prevent viral attachment. Virus Res. 13, 245–261 ( 1989).
Iorio, R.M. et al. Identification of amino-acid residues important to the neuraminidase activity of the HN glycoprotein of Newcastle-disease virus. Virology 173, 196–204 ( 1989).
Sastre, A.G., Cobaleda, C., Cabezas, J.A. & Villar, E. On the inhibition mechanism of the sialidase activity from Newcastle disease virus. Biol. Chem. 372, 923– 927 (1991).
Sagrera, A., Cobaleda, C., Munozbarroso, I., Shnyrov, V. & Villar, E. Modulation of the neuraminidase activity of HN protein from Newcastle disease virus by substrate binding and conformational change: kinetic and thermal denaturation studies. Biochem. Mol. Biol. 37, 717–727 (1995).
Mahon, P.J., Deng, R.T., Mirza, A.M. & Iorio, R.M. Cooperative neuraminidase activity in a paramyxovirus. Virology 213, 241–244 (1995).
Deng, R.T., et al. Mutations in the Newcastle disease virus hemagglutinin-neuraminidase protein that interfere with its ability to interact with the homologous F protein in the promotion of fusion. Virology 253, 43–54 (1999).
Collaborative Computational Project, Number 4. CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994).
Jones, A.T., Zhou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for the building of protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Brunger, A.T., et al. Crystallography andNMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 ( 1996).
Acknowledgements
This work was funded by The Wellcome Trust, the Royal Society, the National Institute of Allergy and Infectious Disease, a Cancer Center Support (CORE) grant, the American Lebanese Syrian Associated charities (ALSAC) of St. Jude Children's Research Hospital and EU TMR/LSF grants for access to the Hamburg and Grenoble synchrotrons. We thank staff at DESY, Hamburg, for their assistance, and the EMBL Grenoble Outstation for providing support for measurements at the ESRF. We thank Walter Ward and Rupert Russell for useful discussions, and Ravi Kartha for carrying out the sialyllactose thin-layer chromatography.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crennell, S., Takimoto, T., Portner, A. et al. Crystal structure of the multifunctional paramyxovirus hemagglutinin-neuraminidase . Nat Struct Mol Biol 7, 1068–1074 (2000). https://doi.org/10.1038/81002
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/81002
This article is cited by
-
The artificial amino acid change in the sialic acid-binding domain of the hemagglutinin neuraminidase of newcastle disease virus increases its specificity to HCT 116 colorectal cancer cells and tumor suppression effect
Virology Journal (2024)
-
Monoclonal antibodies specific for the hemagglutinin-neuraminidase protein define neutralizing epitopes specific for Newcastle disease virus genotype 2.VII from Egypt
Virology Journal (2021)
-
Identification of a potential neutralizing linear epitope of hemagglutinin-neuraminidase in Newcastle disease virus
Virology Journal (2021)
-
Structural basis for Glycan-receptor binding by mumps virus hemagglutinin-neuraminidase
Scientific Reports (2020)
-
Roles of the highly conserved amino acids in the second receptor binding site of the Newcastle disease virus HN protein
Virology Journal (2019)