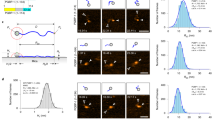
Abstract
Using single protein atomic force microscopy (AFM) techniques we demonstrate that after repeated mechanical extension/relaxation cycles, tandem modular proteins can misfold into a structure formed by two neighboring modules. The misfolding is fully reversible and alters the mechanical topology of the modules while it is about as stable as the original fold. Our results show that modular proteins can assume a novel misfolded state and demonstrate that AFM is able to capture, in real time, rare misfolding events at the level of a single protein.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bork, P., Downing, A.K., Kieffer, B. & Campbell, I.D. Q. Rev. Biophys. 29, 119–167 (1996).
Chothia,C. & Jones, E.Y. Annu. Rev. Biochem. 66, 823–862 ( 1997).
Rief, M., Gautel, M., Oesterhelt, F., Fernandez, J.M. & Gaub, H.E. Science 276, 1109– 1112 (1997).
Oberhauser, A.F., Marszalek, P.E., Erickson, H.P. & Fernandez, J.M. Nature 393, 181–185 ( 1998).
Ohashi, T., Kiehart, D.P. & Erickson, H.P. Proc. Natl. Acad. Sci. USA 96, 2153–2158 (1999).
Erickson, H.P. Science 276, 1090–1092 ( 1997).
Keller, T.C. Nature 387, 233–235 ( 1997).
Hynes, R.O. Proc. Natl. Acad. Sci. 96, 2588–2590 (1999).
Carrion-Vazquez, M. et al. Proc. Natl. Acad. Sci. USA 96, 3694–3699 (1999).
Improta, S., Politou, A.S. & Pastore, A. Structure 4, 323– 337 (1996).
Marko, J.F. & Siggia, E.D. Macromolecules 28, 8759–8770 (1995).
Improta, S. et al. J. Mol. Biol. 284, 761– 777 (1998).
Aukhil, I., Joshi, P., Yan,Y. & Erickson, H.P. J. Biol. Chem. 268, 2542–2552 (1993).
Clark, R.A, Erickson, H.P. & Springer, T.A. J. Cell. Biol. 137, 755– 765 (1997).
Netzer, W. & Hartl, U.F. Nature 388, 343–349 (1997).
Murray, A.J., Lewis, S.J., Barclay, A.N. & Brady, R.L. Proc. Natl. Acad. Sci. USA 92, 7337– 7741 (1995).
Murray, A.J., Head, J.G., Barker, J.J. & Brady, R.L. Nature Struct. Biol. 5, 778–782 ( 1998).
Hayes, M.V., Sessions, R.B., Brady, R.L. & Clarke, A.R. J. Mol. Biol. 285, 1857–1867 (1999).
Evans, E. & Ritchie, K. Biophys. J. 76, 2439–2447 (1999).
Florin, E.L. et al. Biosensors & Bioelectronics 10, 895–901 (1995).
Acknowledgements
We would like to thank H.P. Erickson (Duke University) for providing the human tenascin protein. This work was supported by NIH grants to J.M.F., P.E.M. and A.F.O.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oberhauser, A., Marszalek, P., Carrion-Vazquez, M. et al. Single protein misfolding events captured by atomic force microscopy . Nat Struct Mol Biol 6, 1025–1028 (1999). https://doi.org/10.1038/14907
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/14907
This article is cited by
-
The role of single-protein elasticity in mechanobiology
Nature Reviews Materials (2022)
-
Simulation study for the pulling translocation of a polymer globule
Polymer Journal (2021)
-
A HaloTag-TEV genetic cassette for mechanical phenotyping of proteins from tissues
Nature Communications (2020)
-
Concurrent atomic force spectroscopy
Communications Physics (2019)
-
Investigating the Structural Compaction of Biomolecules Upon Transition to the Gas-Phase Using ESI-TWIMS-MS
Journal of the American Society for Mass Spectrometry (2017)