Abstract
The translational reactivation of maternal mRNAs encoding meiotic drivers in vertebrates is accomplished mainly by cytoplasmic polyadenylation. The cytoplasmic polyadenylation elements (CPEs) present in the 3′ untranslated regions (3′ UTRs) of these transcripts, together with their cognate CPE-binding proteins (CPEBs), define a combinatorial code that determines the timing and extent of translational activation upon meiosis resumption. In addition, the RNA-binding protein Musashi1 (Msi1) regulates polyadenylation of CPE-containing mRNAs by a yet undefined CPEB-dependent or CPEB-independent mechanism. Here we show that Msi1 alone does not support cytoplasmic polyadenylation, but its binding triggers the remodeling of RNA structure, thereby exposing adjacent CPEs and stimulating polyadenylation. In this way, Msi1 directs the preferential use of specific CPEs, which in turn affects the timing and extent of polyadenylation during meiotic progression. Genome-wide analysis of CPEB1- and Msi1-associated mRNAs identified 491 common targets, thus revealing a new layer of CPE-mediated translational control.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Weill, L., Belloc, E., Bava, F.A. & Méndez, R. Translational control by changes in poly(A) tail length: recycling mRNAs. Nat. Struct. Mol. Biol. 19, 577–585 (2012).
Richter, J.D. CPEB: a life in translation. Trends Biochem. Sci. 32, 279–285 (2007).
Coll, O., Villalba, A., Bussotti, G., Notredame, C. & Gebauer, F. A novel, noncanonical mechanism of cytoplasmic polyadenylation operates in Drosophila embryogenesis. Genes Dev. 24, 129–134 (2010).
Kim, K.W. et al. Antagonism between GLD-2 binding partners controls gamete sex. Dev. Cell 16, 723–733 (2009).
Belloc, E. & Méndez, R. A deadenylation negative feedback mechanism governs meiotic metaphase arrest. Nature 452, 1017–1021 (2008).
Eliscovich, C., Peset, I., Vernos, I. & Méndez, R. Spindle-localized CPE-mediated translation controls meiotic chromosome segregation. Nat. Cell Biol. 10, 858–865 (2008).
Piqué, M., López, J.M., Foissac, S., Guigó, R. & Méndez, R. A combinatorial code for CPE-mediated translational control. Cell 132, 434–448 (2008).
Igea, A. & Méndez, R. Meiosis requires a translational positive loop where CPEB1 ensues its replacement by CPEB4. EMBO J. 29, 2182–2193 (2010).
Gunter, K.M. & McLaughlin, E.A. Translational control in germ cell development: A role for the RNA-binding proteins Musashi-1 and Musashi-2. IUBMB Life 63, 678–685 (2011).
Charlesworth, A., Wilczynska, A., Thampi, P., Cox, L.L. & MacNicol, A.M. Musashi regulates the temporal order of mRNA translation during Xenopus oocyte maturation. EMBO J. 25, 2792–2801 (2006).
Arumugam, K., Wang, Y., Hardy, L.L., MacNicol, M.C. & MacNicol, A.M. Enforcing temporal control of maternal mRNA translation during oocyte cell-cycle progression. EMBO J. 29, 387–397 (2010).
Stebbins-Boaz, B., Hake, L.E. & Richter, J.D. CPEB controls the cytoplasmic polyadenylation of cyclin, Cdk2 and c-mos mRNAs and is necessary for oocyte maturation in Xenopus. EMBO J. 15, 2582–2592 (1996).
Charlesworth, A., Ridge, J.A., King, L.A., MacNicol, M.C. & MacNicol, A.M. A novel regulatory element determines the timing of Mos mRNA translation during Xenopus oocyte maturation. EMBO J. 21, 2798–2806 (2002).
Arumugam, K., Macnicol, M.C. & Macnicol, A.M. Autoregulation of Musashi1 mRNA translation during Xenopus oocyte maturation. Mol. Reprod. Dev. 79, 553–563 (2012).
Mendez, R. et al. Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature 404, 302–307 (2000).
Charlesworth, A., Cox, L.L. & MacNicol, A.M. Cytoplasmic polyadenylation element (CPE)- and CPE-binding protein (CPEB)-independent mechanisms regulate early class maternal mRNA translational activation in Xenopus oocytes. J. Biol. Chem. 279, 17650–17659 (2004).
Zearfoss, N.R. et al. A conserved three-nucleotide core motif defines Musashi RNA binding specificity. J. Biol. Chem. 289, 35530–35541 (2014).
Imai, T. et al. The neural RNA-binding protein Musashi1 translationally regulates mammalian numb gene expression by interacting with its mRNA. Mol. Cell. Biol. 21, 3888–3900 (2001).
Curanovic, D. et al. Global profiling of stimulus-induced polyadenylation in cells using a poly(A) trap. Nat. Chem. Biol. 9, 671–673 (2013).
Novoa, I., Gallego, J., Ferreira, P.G. & Mendez, R. Mitotic cell-cycle progression is regulated by CPEB1 and CPEB4-dependent translational control. Nat. Cell Biol. 12, 447–456 (2010).
Ballantyne, S., Daniel, D.L. Jr. & Wickens, M. A dependent pathway of cytoplasmic polyadenylation reactions linked to cell cycle control by c-mos and CDK1 activation. Mol. Biol. Cell 8, 1633–1648 (1997).
Wilkinson, K.A., Merino, E.J. & Weeks, K.M. Selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE): quantitative RNA structure analysis at single nucleotide resolution. Nat. Protoc. 1, 1610–1616 (2006).
Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415 (2003).
Menon, S., Oh, W., Carr, H.S. & Frost, J.A. Rho GTPase-independent regulation of mitotic progression by the RhoGEF Net1. Mol. Biol. Cell 24, 2655–2667 (2013).
Afroz, T. et al. A fly trap mechanism provides sequence-specific RNA recognition by CPEB proteins. Genes Dev. 28, 1498–1514 (2014).
Messias, A.C. & Sattler, M. Structural basis of single-stranded RNA recognition. Acc. Chem. Res. 37, 279–287 (2004).
Rutledge, C.E. et al. Efficient translation of Dnmt1 requires cytoplasmic polyadenylation and Musashi binding elements. PLoS One 9, e88385 (2014).
Battelli, C., Nikopoulos, G.N., Mitchell, J.G. & Verdi, J.M. The RNA-binding protein Musashi-1 regulates neural development through the translational repression of p21WAF-1. Mol. Cell. Neurosci. 31, 85–96 (2006).
Okabe, M., Imai, T., Kurusu, M., Hiromi, Y. & Okano, H. Translational repression determines a neuronal potential in Drosophila asymmetric cell division. Nature 411, 94–98 (2001).
Lagadec, C. et al. The RNA-binding protein Musashi-1 regulates proteasome subunit expression in breast cancer- and glioma-initiating cells. Stem Cells 32, 135–144 (2014).
Kuwako, K. et al. Neural RNA-binding protein Musashi1 controls midline crossing of precerebellar neurons through posttranscriptional regulation of Robo3/Rig-1 expression. Neuron 67, 407–421 (2010).
Katz, Y. et al. Musashi proteins are post-transcriptional regulators of the epithelial-luminal cell state. eLife 3, e03915 (2014).
Planet, E. phenoTest: tools to test association between gene expression and phenotype in a way that is efficient, structured, fast and scalable v.1.24.0 (2013).
Gentleman, R.C. et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80 (2004).
Carvalho, B.S. & Irizarry, R.A. A framework for oligonucleotide microarray preprocessing. Bioinformatics 26, 2363–2367 (2010).
Weill, L. et al. A new type of IRES within gag coding region recruits three initiation complexes on HIV-2 genomic RNA. Nucleic Acids Res. 38, 1367–1381 (2010).
Paris, J. & Richter, J.D. Maturation-specific polyadenylation and translational control: diversity of cytoplasmic polyadenylation elements, influence of poly(A) tail size, and formation of stable polyadenylation complexes. Mol. Cell. Biol. 10, 5634–5645 (1990).
Acknowledgements
We thank the Functional Genomics and Biostatistics/Bioinformatics Facilities at IRB Barcelona. We also thank M. Férnandez, F. Gebauer, B. Sargueil, J. Guillén and members of the Mendez laboratory for useful discussions and/or critical reading of this manuscript. This project was supported by the Fundación Botín and the Banco Santander through its Santander Universities Global Division, the Asociación Española contra el Cáncer, the Worldwide Cancer Research and by the Spanish Ministerio de Economia y Competitividad (BFU2011-30121, BFU2014-54122-P and Consolider RNAREG CSD2009-00080). L.W. was a recipient of an EMBO long-term postdoctoral fellowship. IRB Barcelona is a recipient of a Severo Ochoa Award of Excellence from MINECO (Spanish Government).
Author information
Authors and Affiliations
Contributions
RIP followed by microarray analysis, polyadenylation assays, tethering experiments and secondary structure modeling were performed by L.W. Crosslinking assays, RIP followed by RT-qPCR and SHAPE experiments were conducted by L.W. and E.B. Luciferase experiments were performed by L.W., C.L.C. and E.B. L.W., E.B. and R.M. conceived the study designed the experiments, analyzed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Msi1 targets and interactors.
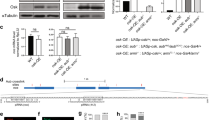
(a) Cyclin B5 mRNA is not an Msi1 target. Immature oocytes were injected first with an mRNA coding for HA-Msi1, then with the indicated reporter. qPCRs of the indicated reporters were performed using reverse transcribed immunoprecipitated mRNAs. Input was normalized against endogenous GAPDH mRNA, and IP efficiency, against endogenous c-mos mRNA. (b) Msi1 and Gld-2 interact in an RNA-dependent manner. Msi1 was immunoprecipitated from immature oocytes (VI), early meiosis (–WS), or after GVBD (+WS). Immature oocytes were injected with HA-Msi1 mRNA, and progesterone was added the next day. When 50% of the oocytes reached GVBD, they were segregated based on whether they had a white spot (+WS) or not (–WS), indicating completed GVBD or not, as previously described (Arumugam et al. EMBO J 29, 387-97, 2010). Oocyte extracts were treated with or without RNase A before HA immunoprecipitation. An anti-HA IP in the absence of HA-Msi1 expression was peformed as a negative control. The presence of HA-Msi1 and Gld-2 was determined by western blot.
Supplementary Figure 2 c-mos 3′ UTR mutational guide.
Scheme showing the c-mos 3′ UTR translation regulatory elements. (b) Name of c-mos constructs used in the mutational study. Descriptions of the regulatory elements mutations, followed by description of the schematic representations depicted in Fig. 3 are shown.
Supplementary Figure 3 c-mos mutant variants study; MBE and CPE mutations effect on Msi1 binding and translation activation.
Immature oocytes were injected with the indicated RNA constructs, then overexpressed HA-Msi1 or endogenous CPEB1 were immnoprecipitated. The IPs were analyzed by western blot and by RT qPCR. Anti-HA IP without HA-Msi1 overexpression and IgG IP were used as controls. (a) Left panel, IP HA-Msi1 analyzed by western blot. Right panel, qPCRs of the indicated reporters performed using reversed transcribed immunoprecipitated mRNAs. Input was normalized against endogenous GAPDH mRNA, and IP efficiency, against endogenous c-mos mRNA. (b) Left panel, CPEB1 IP analyzed by western blot; right panel, qPCR of the indicated reporter after reverse transcription of the immunoprecipitated mRNAs (data, mean ± SEM; n = 3). (c) Translational regulation of Mos (+12) and non-consensus CPE containing reporters. Synthetic mRNAs containing the firefly luciferase coding sequence fused to the indicated 3′ UTRs were co-injected with control renilla luciferase mRNA. Oocytes were collected 2 h after GVBD to determine luciferase activity. Schematic representations of each 3′ UTR are shown to the left, with consensus CPEs (C) depicted as blue boxes; non-consensus CPEs, green boxes; MBEs (M), orange circles; PAS (H), red hexagons; and mutated CPE, MBE or PAS, as an empty box, circle or hexagon, respectively; distance between elements is given in nucleotides.
Supplementary Figure 4 c-mos MBE mutant study; CPEB1 binding of MBEb mutant, and SHAPE histograms.
(a) Effects of MBEb mutation on CPEB1 binding. Immature oocytes were injected with the indicated constructs. Left panel, IP against CPEB1 analyzed by western blot; right panel, qPCR of the indicated reporter after reverse transcription of the immunoprecipitated mRNAs (data, mean ± SEM; n = 3). (b) Absolute SHAPE reactivities of WT Mos and Mos MBE mutant variants. The intensity of RT stops was measured at each nucleotide in the absence or presence of NMIA with ImageJ. The absolute SHAPE reactivity was obtained by substracting the intensity in the control (–NMIA) from that in presence of NMIA, and then normalized to the intensity control. A nucleotide was considered reactive (green bars) when its SHAPE reactivity was > 1.3-fold threshold (green dashed line). To model the secondary structure of these transcripts, only the nucleotides with a SHAPE reactivity above 3-fold were assigned as single-stranded in the Mfold software. Since no differences were observed in the upstream regions, for Mos[–MBEa] only the regions from nucleotides 47–109 are shown and for the other mutant variants nucleotides 62–109 are shown.
Supplementary Figure 5 Mos[WT] RNA structure remodeling upon Msi1 binding.
(a) Pulled down Gst-Msi1 by affinity chromatography (glutathione magnetic beads) analyzed by SDS-PAGE and coomassie staining. (b) Absolute SHAPE reactivities of Mos[WT] in the presence of Msi1. The absolute SHAPE reactivity was measured as described in Supplementary Fig. 4. In the absence of Msi1 (solid bars), the SHAPE reactivity for Mos[WT] was calculated with the control lane without Msi1 (–NMIA, –Msi1), while the SHAPE reactivity in the presence of Msi1 (red bar) was calculated with the control lane (–NMIA, +Msi1). The reactivity threshold (1.3-fold) is indicated as a green dotted line. Increased NMIA sensitivity due to Msi1 binding is indicated in red (+Msi1), and decreased NMIA sensibility due to the presence of Msi1 is indicated in green (–Msi). (c) Mos[WT] 3′ UTR experimentally validated secondary structure after Msi1 binding. Green circles correspond to nucleotides modified by NMIA in either the presence or absence of Msi1. Red circles correspond to nucleotides modified by NMIA only in the presence of Msi1. Green squares correspond to nucleotides modified by NMIA only in the absence of Msi1 (i.e., protected by Msi1 from modification). This Msi1 protection is probably due to direct binding of Msi1 to the mRNA. Only nucleotides with an absolute SHAPE reactivity above 3-fold were assigned as single-stranded in Mfold prediction software.
Supplementary Figure 6 Structure mutations secondary structure and CPEB1 binding study.
(a) SHAPE. Chemical probing of RNA secondary structures with NMIA in the presence of Mg2+. Denaturing TBE-PAGE gel with Mos[WT] sequencing reaction next to NMIA probing. The nucleotide position corresponding to the dideoxynucleotide sequence is indicated at the left. (b) Absolute SHAPE reactivities from panel a. Analyzed as described in Supplementary Fig. 4. (c) UV-Crosslinking of c-mos RNA probes. Upper panel: stage VI extracts incubated with the indicated 3′ UTR radioactive probes, UV-crosslinked, digested with RNase A, and visualized by autoradiography after 10% SDS-PAGE. Lower panel: commassie staining of the 10% SDS-PAGE gel used for autoradiography. Uncropped blot images are shown in Supplementary Data Set 3.
Supplementary Figure 7 net1 mRNA a new CPEB1 and Msi1 target.
RT-qPCR after CPEB1 or HA-Msi1 immunoprecipitation. Left panel: RTqPCRs of the indicated endogenous mRNAs were performed using reverse-transcribed, CPEB1-immunoprecipitated mRNAs. Right Panel: Immature oocytes were first injected with an mRNA encoding HA-Msi1. RT-qPCRs of the indicated endogenous mRNAs were then performed using reverse-transcribed, HA-immunoprecipitated mRNAs. Input was normalized against endogenous GAPDH mRNA, and IP efficiency, against endogenous c-mos mRNA. n=2 of independent experiments. (b) SHAPE. Chemical probing of RNA secondary structures with NMIA in the presence of Mg2+. Denaturing TBE-PAGE gels with Net1[WT] sequencing reaction next to NMIA probing. The nucleotide position corresponding to the dideoxynucleotide sequence is indicated to the left. n = 2 of independent experiments. Uncropped images are shown in Supplementary Data Set 3.
Supplementary Figure 8 Absolute SHAPE reactivities for Net1 transcripts.
The intensity of RT stops were measured as described in Supplementary Fig. 4. A nucleotide was considered reactive (green bar) when its SHAPE reactivity was above the 1.3-fold threshold (indicated as a green dashed line). To model the secondary structure of these transcripts, only nucleotides with a SHAPE reactivity above 3-fold were assigned as single-stranded in the Mfold prediction software
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1. (PDF 1868 kb)
Supplementary Data Set 1
CPEB1 and HA-MSI RIP Microarray result. (XLSX 4931 kb)
Supplementary Data Set 2
CPEB1 and Msi1 polyadenylated targets. (XLSX 21 kb)
Supplementary Data Set 3
Uncropped blot images. (PDF 38404 kb)
Reporting Summary
Life Sciences Reporting Summary. (PDF 129 kb)
Rights and permissions
About this article
Cite this article
Weill, L., Belloc, E., Castellazzi, C. et al. Musashi 1 regulates the timing and extent of meiotic mRNA translational activation by promoting the use of specific CPEs. Nat Struct Mol Biol 24, 672–681 (2017). https://doi.org/10.1038/nsmb.3434
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3434
This article is cited by
-
The molecular regulatory mechanisms of meiotic arrest and resumption in Oocyte development and maturation
Reproductive Biology and Endocrinology (2023)
-
Dueling RNA-binding proteins promote translational activation
Nature Structural & Molecular Biology (2017)