Abstract
Hypertrophic cardiomyopathy (HCM) is primarily caused by mutations in β-cardiac myosin and myosin-binding protein-C (MyBP-C). Changes in the contractile parameters of myosin measured so far do not explain the clinical hypercontractility caused by such mutations. We propose that hypercontractility is due to an increase in the number of myosin heads (S1) that are accessible for force production. In support of this hypothesis, we demonstrate myosin tail (S2)-dependent functional regulation of actin-activated human β-cardiac myosin ATPase. In addition, we show that both S2 and MyBP-C bind to S1 and that phosphorylation of either S1 or MyBP-C weakens these interactions. Importantly, the S1-S2 interaction is also weakened by four myosin HCM-causing mutations but not by two other mutations. To explain these experimental results, we propose a working structural model involving multiple interactions, including those with myosin's own S2 and MyBP-C, that hold myosin in a sequestered state.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Woodhead, J.L. et al. Atomic model of a myosin filament in the relaxed state. Nature 436, 1195–1199 (2005).
Alamo, L. et al. Three-dimensional reconstruction of tarantula myosin filaments suggests how phosphorylation may regulate myosin activity. J. Mol. Biol. 384, 780–797 (2008).
Zhao, F.Q., Craig, R. & Woodhead, J.L. Head-head interaction characterizes the relaxed state of Limulus muscle myosin filaments. J. Mol. Biol. 385, 423–431 (2009).
Pinto, A., Sánchez, F., Alamo, L. & Padrón, R. The myosin interacting-heads motif is present in the relaxed thick filament of the striated muscle of scorpion. J. Struct. Biol. 180, 469–478 (2012).
Alamo, L. et al. Conserved intramolecular interactions maintain myosin interacting-heads motifs explaining tarantula muscle super-relaxed state structural basis. J. Mol. Biol. 428, 1142–1164 (2016).
Woodhead, J.L., Zhao, F.Q. & Craig, R. Structural basis of the relaxed state of a Ca2+-regulated myosin filament and its evolutionary implications. Proc. Natl. Acad. Sci. USA 110, 8561–8566 (2013).
Zoghbi, M.E., Woodhead, J.L., Moss, R.L. & Craig, R. Three-dimensional structure of vertebrate cardiac muscle myosin filaments. Proc. Natl. Acad. Sci. USA 105, 2386–2390 (2008).
Al-Khayat, H.A., Kensler, R.W., Squire, J.M., Marston, S.B. & Morris, E.P. Atomic model of the human cardiac muscle myosin filament. Proc. Natl. Acad. Sci. USA 110, 318–323 (2013).
González-Solá, M., Al-Khayat, H.A., Behra, M. & Kensler, R.W. Zebrafish cardiac muscle thick filaments: isolation technique and three-dimensional structure. Biophys. J. 106, 1671–1680 (2014).
Nogales, E. & Scheres, S.H. Cryo-EM: a unique tool for the visualization of macromolecular complexity. Mol. Cell 58, 677–689 (2015).
Cheng, Y. Single-particle cryo-EM at crystallographic resolution. Cell 161, 450–457 (2015).
Bai, X.C., McMullan, G. & Scheres, S.H. How cryo-EM is revolutionizing structural biology. Trends Biochem. Sci. 40, 49–57 (2015).
Henderson, R. Realizing the potential of electron cryo-microscopy. Q. Rev. Biophys. 37, 3–13 (2004).
Hu, Z., Taylor, D.W., Reedy, M.K., Edwards, R.J. & Taylor, K.A. Structure of myosin filaments from relaxed Lethocerus flight muscle by cryo-EM at 6 Å resolution. Sci. Adv. 2, e1600058 (2016).
Maron, B.J. & Maron, M.S. Hypertrophic cardiomyopathy. Lancet 381, 242–255 (2013).
Harvey, P.A. & Leinwand, L.A. The cell biology of disease: cellular mechanisms of cardiomyopathy. J. Cell Biol. 194, 355–365 (2011).
Buvoli, M., Hamady, M., Leinwand, L.A. & Knight, R. Bioinformatics assessment of β-myosin mutations reveals myosin's high sensitivity to mutations. Trends Cardiovasc. Med. 18, 141–149 (2008).
Walsh, R., Rutland, C., Thomas, R. & Loughna, S. Cardiomyopathy: a systematic review of disease-causing mutations in myosin heavy chain 7 and their phenotypic manifestations. Cardiology 115, 49–60 (2010).
Colegrave, M. & Peckham, M. Structural implications of β-cardiac myosin heavy chain mutations in human disease. Anat. Rec. (Hoboken) 297, 1670–1680 (2014).
Alcalai, R., Seidman, J.G. & Seidman, C.E. Genetic basis of hypertrophic cardiomyopathy: from bench to the clinics. J. Cardiovasc. Electrophysiol. 19, 104–110 (2008).
Seidman, J.G. & Seidman, C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell 104, 557–567 (2001).
Seidman, C.E. & Seidman, J.G. Hypertrophic cardiomyopathy. in The Metabolic and Molecular Bases of Inherited Disease (eds. Scriver, C.R. et al.) 5532–5452 (McGraw-Hill, 2000).
Tyska, M.J. et al. Single-molecule mechanics of R403Q cardiac myosin isolated from the mouse model of familial hypertrophic cardiomyopathy. Circ. Res. 86, 737–744 (2000).
Spudich, J.A. Hypertrophic and dilated cardiomyopathy: four decades of basic research on muscle lead to potential therapeutic approaches to these devastating genetic diseases. Biophys. J. 106, 1236–1249 (2014).
Adhikari, A.S. et al. Early-onset hypertrophic cardiomyopathy mutations significantly increase the velocity, force, and actin-activated ATPase activity of human β-cardiac myosin. Cell Rep. 17, 2857–2864 (2016).
Nag, S. et al. Contractility parameters of human β-cardiac myosin with the hypertrophic cardiomyopathy mutation R403Q show loss of motor function. Sci. Adv. 1, e1500511 (2015).
Sommese, R.F. et al. Molecular consequences of the R453C hypertrophic cardiomyopathy mutation on human β-cardiac myosin motor function. Proc. Natl. Acad. Sci. USA 110, 12607–12612 (2013).
Homburger, J.R. et al. Multidimensional structure-function relationships in human β-cardiac myosin from population-scale genetic variation. Proc. Natl. Acad. Sci. USA 113, 6701–6706 (2016).
Kawana, M., Sarkar, S.S., Sutton, S., Ruppel, K.M. & Spudich, J.A. Biophysical properties of human β-cardiac myosin with converter mutations that cause hypertrophic cardiomyopathy. Sci. Adv. 3, e1601959 (2017).
Kampourakis, T. & Irving, M. Phosphorylation of myosin regulatory light chain controls myosin head conformation in cardiac muscle. J. Mol. Cell. Cardiol. 85, 199–206 (2015).
Stewart, M.A., Franks-Skiba, K., Chen, S. & Cooke, R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc. Natl. Acad. Sci. USA 107, 430–435 (2010).
Hooijman, P., Stewart, M.A. & Cooke, R. A new state of cardiac myosin with very slow ATP turnover: a potential cardioprotective mechanism in the heart. Biophys. J. 100, 1969–1976 (2011).
Brito, R. et al. A molecular model of phosphorylation-based activation and potentiation of tarantula muscle thick filaments. J. Mol. Biol. 414, 44–61 (2011).
Blankenfeldt, W., Thomä, N.H., Wray, J.S., Gautel, M. & Schlichting, I. Crystal structures of human cardiac beta-myosin II S2-Delta provide insight into the functional role of the S2 subfragment. Proc. Natl. Acad. Sci. USA 103, 17713–17717 (2006).
Moore, J.R., Leinwand, L. & Warshaw, D.M. Understanding cardiomyopathy phenotypes based on the functional impact of mutations in the myosin motor. Circ. Res. 111, 375–385 (2012).
Spudich, J.A. The myosin mesa and a possible unifying hypothesis for the molecular basis of human hypertrophic cardiomyopathy. Biochem. Soc. Trans. 43, 64–72 (2015).
Spudich, J.A. et al. Effects of hypertrophic and dilated cardiomyopathy mutations on power output by human β-cardiac myosin. J. Exp. Biol. 219, 161–167 (2016).
Craig, R. & Offer, G. The location of C-protein in rabbit skeletal muscle. Proc. R. Soc. Lond. B Biol. Sci. 192, 451–461 (1976).
Wendt, T., Taylor, D., Trybus, K.M. & Taylor, K. Three-dimensional image reconstruction of dephosphorylated smooth muscle heavy meromyosin reveals asymmetry in the interaction between myosin heads and placement of subfragment 2. Proc. Natl. Acad. Sci. USA 98, 4361–4366 (2001).
Burgess, S.A. et al. Structures of smooth muscle myosin and heavy meromyosin in the folded, shutdown state. J. Mol. Biol. 372, 1165–1178 (2007).
Jung, H.S., Komatsu, S., Ikebe, M. & Craig, R. Head-head and head-tail interaction: a general mechanism for switching off myosin II activity in cells. Mol. Biol. Cell 19, 3234–3242 (2008).
Jung, H.S. et al. Role of the tail in the regulated state of myosin 2. J. Mol. Biol. 408, 863–878 (2011).
Jung, H.S. et al. Conservation of the regulated structure of folded myosin 2 in species separated by at least 600 million years of independent evolution. Proc. Natl. Acad. Sci. USA 105, 6022–6026 (2008).
Nag, S. et al. Beyond the myosin mesa: a potential unifying hypothesis on the underlying molecular basis of hyper-contractility caused by a majority of hypertrophic cardiomyopathy mutations. Preprint at http://biorxiv.org/content/early/2016/07/24/065508/ (2016).
Winkelmann, D.A., Forgacs, E., Miller, M.T. & Stock, A.M. Structural basis for drug-induced allosteric changes to human β-cardiac myosin motor activity. Nat. Commun. 6, 7974 (2015).
Scruggs, S.B. & Solaro, R.J. The significance of regulatory light chain phosphorylation in cardiac physiology. Arch. Biochem. Biophys. 510, 129–134 (2011).
Toepfer, C. et al. Myosin regulatory light chain (RLC) phosphorylation change as a modulator of cardiac muscle contraction in disease. J. Biol. Chem. 288, 13446–13454 (2013).
Levine, R.J., Kensler, R.W., Yang, Z., Stull, J.T. & Sweeney, H.L. Myosin light chain phosphorylation affects the structure of rabbit skeletal muscle thick filaments. Biophys. J. 71, 898–907 (1996).
Trybus, K.M., Freyzon, Y., Faust, L.Z. & Sweeney, H.L. Spare the rod, spoil the regulation: necessity for a myosin rod. Proc. Natl. Acad. Sci. USA 94, 48–52 (1997).
Guo, R. et al. BS69/ZMYND11 reads and connects histone H3.3 lysine 36 trimethylation-decorated chromatin to regulated pre-mRNA processing. Mol. Cell 56, 298–310 (2014).
Guo, Y., Scheuermann, T.H., Partch, C.L., Tomchick, D.R. & Gardner, K.H. Coiled-coil coactivators play a structural role mediating interactions in hypoxia-inducible factor heterodimerization. J. Biol. Chem. 290, 7707–7721 (2015).
van den Bogaart, G., Meyenberg, K., Diederichsen, U. & Jahn, R. Phosphatidylinositol 4,5-bisphosphate increases Ca2+ affinity of synaptotagmin-1 by 40-fold. J. Biol. Chem. 287, 16447–16453 (2012).
Gruen, M. & Gautel, M. Mutations in beta-myosin S2 that cause familial hypertrophic cardiomyopathy (FHC) abolish the interaction with the regulatory domain of myosin-binding protein-C. J. Mol. Biol. 286, 933–949 (1999).
Ratti, J., Rostkova, E., Gautel, M. & Pfuhl, M. Structure and interactions of myosin-binding protein C domain C0: cardiac-specific regulation of myosin at its neck? J. Biol. Chem. 286, 12650–12658 (2011).
Kaski, J.P. et al. Prevalence of sarcomere protein gene mutations in preadolescent children with hypertrophic cardiomyopathy. Circ. Cardiovasc. Genet. 2, 436–441 (2009).
Morita, H. et al. Shared genetic causes of cardiac hypertrophy in children and adults. N. Engl. J. Med. 358, 1899–1908 (2008).
Naber, N., Cooke, R. & Pate, E. Slow myosin ATP turnover in the super-relaxed state in tarantula muscle. J. Mol. Biol. 411, 943–950 (2011).
Nogara, L. et al. Spectroscopic studies of the super relaxed state of skeletal muscle. PLoS One 11, e0160100 (2016).
Cremo, C.R., Sellers, J.R. & Facemyer, K.C. Two heads are required for phosphorylation-dependent regulation of smooth muscle myosin. J. Biol. Chem. 270, 2171–2175 (1995).
Harris, S.P., Lyons, R.G. & Bezold, K.L. In the thick of it: HCM-causing mutations in myosin binding proteins of the thick filament. Circ. Res. 108, 751–764 (2011).
Helms, A.S. et al. Sarcomere mutation-specific expression patterns in human hypertrophic cardiomyopathy. Circ. Cardiovasc. Genet. 7, 434–443 (2014).
Marston, S. et al. Evidence from human myectomy samples that MYBPC3 mutations cause hypertrophic cardiomyopathy through haploinsufficiency. Circ. Res. 105, 219–222 (2009).
Yang, Y. et al. Rigor-like structures from muscle myosins reveal key mechanical elements in the transduction pathways of this allosteric motor. Structure 15, 553–564 (2007).
von der Ecken, J., Heissler, S.M., Pathan-Chhatbar, S., Manstein, D.J. & Raunser, S. Cryo-EM structure of a human cytoplasmic actomyosin complex at near-atomic resolution. Nature 534, 724–728 (2016).
Behrmann, E. et al. Structure of the rigor actin-tropomyosin-myosin complex. Cell 150, 327–338 (2012).
Rayment, I. et al. Structure of the actin-myosin complex and its implications for muscle contraction. Science 261, 58–65 (1993).
Schröder, R.R. et al. Three-dimensional atomic model of F-actin decorated with Dictyostelium myosin S1. Nature 364, 171–174 (1993).
Huang, J., Koide, A., Makabe, K. & Koide, S. Design of protein function leaps by directed domain interface evolution. Proc. Natl. Acad. Sci. USA 105, 6578–6583 (2008).
Siemankowski, R.F. & White, H.D. Kinetics of the interaction between actin, ADP, and cardiac myosin-S1. J. Biol. Chem. 259, 5045–5053 (1984).
Rybakova, I.N., Greaser, M.L. & Moss, R.L. Myosin binding protein C interaction with actin: characterization and mapping of the binding site. J. Biol. Chem. 286, 2008–2016 (2011).
Jia, W., Shaffer, J.F., Harris, S.P. & Leary, J.A. Identification of novel protein kinase A phosphorylation sites in the M-domain of human and murine cardiac myosin binding protein-C using mass spectrometry analysis. J. Proteome Res. 9, 1843–1853 (2010).
UniProt Consortium. Update on activities at the Universal Protein Resource (UniProt) in 2013. Nucleic Acids Res. 41, D43–D47 (2013).
Syamaladevi, D.P. et al. Myosinome: a database of myosins from select eukaryotic genomes to facilitate analysis of sequence-structure-function relationships. Bioinform. Biol. Insights 6, 247–254 (2012).
Xu, D. & Zhang, Y. Ab initio protein structure assembly using continuous structure fragments and optimized knowledge-based force field. Proteins 80, 1715–1735 (2012).
Yang, J. et al. The I-TASSER Suite: protein structure and function prediction. Nat. Methods 12, 7–8 (2015).
Lovell, S.C. et al. Structure validation by Calpha geometry: phi,psi and Cbeta deviation. Proteins 50, 437–450 (2003).
Duhr, S. & Braun, D. Why molecules move along a temperature gradient. Proc. Natl. Acad. Sci. USA 103, 19678–19682 (2006).
Seidel, S.A. et al. Microscale thermophoresis quantifies biomolecular interactions under previously challenging conditions. Methods 59, 301–315 (2013).
Pardee, J.D. & Spudich, J.A. Purification of muscle actin. Methods Enzymol. 85, Pt B 164–181 (1982).
Trybus, K.M. Biochemical studies of myosin. Methods 22, 327–335 (2000).
De La Cruz, E.M. & Ostap, E.M. Kinetic and equilibrium analysis of the myosin ATPase. Methods Enzymol. 455, 157–192 (2009).
Efron, B. The Jackknife, the Bootstrap, and other Resampling Plans Vol 2 (Society for Industrial and Applied Mathematics, 1982).
Acknowledgements
We thank A. Houdusse for helpful suggestions regarding the myosin model. We thank A. Borrayo of the laboratory of J.A.S. for technical help in maintaining virus and cell stocks. We also thank the members of NanoTemper Technologies (South San Francisco) for training us on the MST instrument and analysis. This work was funded by NIH grants GM33289 and HL117138 to J.A.S.; a Stanford Lucile Packard CHRI Postdoctoral Award (UL1 TR001085) and an American Heart Association Postdoctoral Fellowship (17POST33411070) to D.V.T.; and a Stanford Lucile Packard CHRI Postdoctoral Award (UL1 TR001085), a Stanford ChEM-H Postdocs at the Interface Award, and an American Heart Association Postdoctoral Fellowship (16POST30890005) to A.S.A.
Author information
Authors and Affiliations
Contributions
Molecular modeling studies were performed by J.A.S. and M.S.S. All binding experiments were performed by S.N. and D.V.T., and those involving the 2-hep HMM were performed with help from S.S.S. and K.M.R. S.N., D.V.T., and J.A.S. contributed to data analysis and interpretation. A.S.A. purified the R249Q protein and performed the MST with D.V.T. All ATPase data were performed and analyzed by A.S.A. with help from S.S.S. for reagent production. S.N., D.V.T., and J.A.S. wrote the manuscript. S.N., D.V.T., S.S.S., A.S.A., K.M.R., and J.A.S. contributed to editing of the final manuscript. S.N., D.V.T., S.S.S., A.S.A., S.S., and K.M.R. contributed to the development of new reagents.
Corresponding authors
Ethics declarations
Competing interests
J.A.S. is a founder of Cytokinetics and MyoKardia and a member of their scientific advisory boards.
Integrated supplementary information
Supplementary Figure 1 Binding of sS1 to proximal S2 using MST.
Binding of Cy5-tagged sS1 to proximal S2 at 25 mM (orange) and 100 mM (black) KCl. All data shown in the figure are representative curves; data points are mean and s.e.m. from n=3 readings from a single protein preparation; source data are in Supplementary Table 2. Data from multiple preparations are summarized in Supplementary Table 1.
Supplementary Figure 2 Binding of 2-hep HMM to proximal S2 at 100 mM KCl using MST.
Non-phosphorylated (-Phos) and phosphorylated (+Phos) 2-hep HMM are compared. All data shown in the figure are representative data points are mean and s.e.m. from n=3 readings from a single protein preparation; source data are in Supplementary Table 2.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–2, Supplementary Table 1 and Supplementary Note 1 (PDF 801 kb)
Supplementary Data Set 1
Basic folded back model for human cardiac b-myosin. (PDB 3292 kb)
Supplementary Data Set 2
Folded back model with myosin binding protein C fragment C0-C2 modeled in. (PDB 3865 kb)
Supplementary Data Set 3
Folded back model with full length myosin binding protein C modeled in. (PDB 4865 kb)
Three faces of the myosin catalytic domain.
The three distinct regions of the myosin catalytic domain are highlighted as myosin mesa (pink), actin binding interface (yellow) and the blocked-head converter binding face (dark gray with red residue in middle). The blue residues are the mesa HCM-causing mutations. Refer text for further details. (MOV 30395 kb)
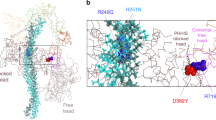
The myosin mesa-proximal S2 interface and the location of HCM-causing mutations.
Homology modelled folded-back human β-cardiac myosin is shown. The pink spheres in the catalytic domain is the mesa region, and the blue residues are HCM-causing mutations. Note the close proximity of these HCM-causing mesa residues to the coiled-coil proximal S2. HCM-causing mutations on S2 are shown as red spheres. Refer text for further details. (MOV 42654 kb)
Rights and permissions
About this article
Cite this article
Nag, S., Trivedi, D., Sarkar, S. et al. The myosin mesa and the basis of hypercontractility caused by hypertrophic cardiomyopathy mutations. Nat Struct Mol Biol 24, 525–533 (2017). https://doi.org/10.1038/nsmb.3408
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3408
This article is cited by
-
Discovery of a novel cardiac-specific myosin modulator using artificial intelligence-based virtual screening
Nature Communications (2023)
-
Cryo-EM structure of the folded-back state of human β-cardiac myosin
Nature Communications (2023)
-
Hypertrophic cardiomyopathy in purpose-bred cats with the A31P mutation in cardiac myosin binding protein-C
Scientific Reports (2023)
-
Cryo-EM structure of the human cardiac myosin filament
Nature (2023)
-
Etiology of genetic muscle disorders induced by mutations in fast and slow skeletal MyBP-C paralogs
Experimental & Molecular Medicine (2023)