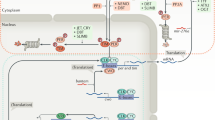
Abstract
Circadian clocks are cell-autonomous oscillators regulating daily rhythms in a wide range of physiological, metabolic and behavioral processes. Feedback of metabolic signals, such as redox state, NAD+/NADH and AMP/ADP ratios, or heme, modulate circadian rhythms and thereby optimize energy utilization across the 24-h cycle. We show that rhythmic heme degradation, which generates the signaling molecule carbon monoxide (CO), is required for normal circadian rhythms as well as circadian metabolic outputs. CO suppresses circadian transcription by attenuating CLOCK–BMAL1 binding to target promoters. Pharmacological inhibition or genetic depletion of CO-producing heme oxygenases abrogates normal daily cycles in mammalian cells and Drosophila. In mouse hepatocytes, suppression of CO production leads to a global upregulation of CLOCK–BMAL1-dependent circadian gene expression and dysregulated glucose metabolism. Together, our findings show that CO metabolism is an important link between the basic circadian-clock machinery, metabolism and behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Buhr, E.D. & Takahashi, J.S. Molecular components of the mammalian circadian clock. Handb. Exp. Pharmacol. 217, 3–27 (2013).
Asher, G. & Schibler, U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 13, 125–137 (2011).
Yin, L., Wu, N. & Lazar, M.A. Nuclear receptor Rev-erbalpha: a heme receptor that coordinates circadian rhythm and metabolism. Nucl. Recept. Signal. 8, e001 (2010).
Yin, L. et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 318, 1786–1789 (2007).
Raghuram, S. et al. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nat. Struct. Mol. Biol. 14, 1207–1213 (2007).
Dioum, E.M. et al. NPAS2: a gas-responsive transcription factor. Science 298, 2385–2387 (2002).
Oliveri, L.M., Davio, C., Batlle, A.M. & Gerez, E.N. ALAS1 gene expression is down-regulated by Akt-mediated phosphorylation and nuclear exclusion of FOXO1 by vanadate in diabetic mice. Biochem. J. 442, 303–310 (2012).
Kaasik, K. & Lee, C.C. Reciprocal regulation of haem biosynthesis and the circadian clock in mammals. Nature 430, 467–471 (2004).
Abraham, N.G. & Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 60, 79–127 (2008).
Martasek, P. et al. Properties of human kidney heme oxygenase: inhibition by synthetic heme analogues and metalloporphyrins. Biochem. Biophys. Res. Commun. 157, 480–487 (1988).
Sardana, M.K. & Kappas, A. Dual control mechanism for heme oxygenase: tin(IV)-protoporphyrin potently inhibits enzyme activity while markedly increasing content of enzyme protein in liver. Proc. Natl. Acad. Sci. USA 84, 2464–2468 (1987).
Xu, Y.Q. et al. Diurnal variation of hepatic antioxidant gene expression in mice. PLoS One 7, e44237 (2012).
Ryter, S.W., Alam, J. & Choi, A.M. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 86, 583–650 (2006).
Yet, S.F. et al. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J. Clin. Invest. 103, R23–R29 (1999).
Satarug, S. & Moore, M.R. Emerging roles of cadmium and heme oxygenase in type-2 diabetes and cancer susceptibility. Tohoku J. Exp. Med. 228, 267–288 (2012).
Hughes, M.E. et al. Harmonics of circadian gene transcription in mammals. PLoS Genet. 5, e1000442 (2009).
Rey, G. et al. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol. 9, e1000595 (2011).
Cho, H. et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485, 123–127 (2012).
Ceriani, M.F. et al. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J. Neurosci. 22, 9305–9319 (2002).
Ueda, H.R. et al. Genome-wide transcriptional orchestration of circadian rhythms in Drosophila. J. Biol. Chem. 277, 14048–14052 (2002).
Cui, L. et al. Relevant expression of Drosophila heme oxygenase is necessary for the normal development of insect tissues. Biochem. Biophys. Res. Commun. 377, 1156–1161 (2008).
Brand, A.H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Hamblen-Coyle, M.J., Wheeler, D.A., Rutila, J.E., Rosbash, M. & Hall, J.C. Behavior of period-altered circadian rhythm mutants of Drosophila in light:dark cycles. J. Insect Behav. 5, 417–446 (1992).
Kaneko, M. & Hall, J.C. Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J. Comp. Neurol. 422, 66–94 (2000).
Grima, B., Chélot, E., Xia, R. & Rouyer, F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature 431, 869–873 (2004).
Park, J.H. & Hall, J.C. Isolation and chronobiological analysis of a neuropeptide pigment-dispersing factor gene in Drosophila melanogaster. J. Biol. Rhythms 13, 219–228 (1998).
Stoleru, D., Peng, Y., Agosto, J. & Rosbash, M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 431, 862–868 (2004).
Mandilaras, K. & Missirlis, F. Genes for iron metabolism influence circadian rhythms in Drosophila melanogaster. Metallomics 4, 928–936 (2012).
Stanewsky, R., Jamison, C.F., Plautz, J.D., Kay, S.A. & Hall, J.C. Multiple circadian-regulated elements contribute to cycling period gene expression in Drosophila. EMBO J. 16, 5006–5018 (1997).
Veleri, S., Brandes, C., Helfrich-Förster, C., Hall, J.C. & Stanewsky, R. A self-sustaining, light-entrainable circadian oscillator in the Drosophila brain. Curr. Biol. 13, 1758–1767 (2003).
Lukat-Rodgers, G.S., Correia, C., Botuyan, M.V., Mer, G. & Rodgers, K.R. Heme-based sensing by the mammalian circadian protein CLOCK. Inorg. Chem. 49, 6349–6365 (2010).
Ingi, T., Cheng, J. & Ronnett, G.V. Carbon monoxide: an endogenous modulator of the nitric oxide-cyclic GMP signaling system. Neuron 16, 835–842 (1996).
Ishida, M., Ueha, T. & Sagami, I. Effects of mutations in the heme domain on the transcriptional activity and DNA-binding activity of NPAS2. Biochem. Biophys. Res. Commun. 368, 292–297 (2008).
Rey, G. et al. The pentose phosphate pathway regulates the circadian clock. Cell Metab. 24, 462–473 (2016).
Kim, H.P., Ryter, S.W. & Choi, A.M. CO as a cellular signaling molecule. Annu. Rev. Pharmacol. Toxicol. 46, 411–449 (2006).
Artinian, L.R., Ding, J.M. & Gillette, M.U. Carbon monoxide and nitric oxide: interacting messengers in muscarinic signaling to the brain's circadian clock. Exp. Neurol. 171, 293–300 (2001).
Rubio, M.F., Agostino, P.V., Ferreyra, G.A. & Golombek, D.A. Circadian heme oxygenase activity in the hamster suprachiasmatic nuclei. Neurosci. Lett. 353, 9–12 (2003).
Lira, V.A. et al. Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 293, E1062–E1068 (2007).
Lamia, K.A. et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326, 437–440 (2009).
Cool, B. et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 3, 403–416 (2006).
Shao, D. et al. A redox-dependent mechanism for regulation of AMPK activation by Thioredoxin1 during energy starvation. Cell Metab. 19, 232–245 (2014).
Viollet, B. et al. AMPK: lessons from transgenic and knockout animals. Front. Biosci. (Landmark Ed.) 14, 19–44 (2009).
Yoshii, T., Rieger, D. & Helfrich-Förster, C. Two clocks in the brain: an update of the morning and evening oscillator model in Drosophila. Prog. Brain. Res. 199, 59–82 (2012).
Yoshii, T. et al. The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila's clock. J. Neurosci. 29, 2597–2610 (2009).
Sodhi, K. et al. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J. Pharmacol. Exp. Ther. 331, 906–916 (2009).
Jais, A. et al. Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell 158, 25–40 (2014).
Chen, S., Khan, Z.A., Barbin, Y. & Chakrabarti, S. Pro-oxidant role of heme oxygenase in mediating glucose-induced endothelial cell damage. Free Radic. Res. 38, 1301–1310 (2004).
Paredi, P., Biernacki, W., Invernizzi, G., Kharitonov, S.A. & Barnes, P.J. Exhaled carbon monoxide levels elevated in diabetes and correlated with glucose concentration in blood: a new test for monitoring the disease? Chest 116, 1007–1011 (1999).
Sandrelli, F. et al. A molecular basis for natural selection at the timeless locus in Drosophila melanogaster. Science 316, 1898–1900 (2007).
Bischof, J., Maeda, R.K., Hediger, M., Karch, F. & Basler, K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104, 3312–3317 (2007).
Renn, S.C., Park, J.H., Rosbash, M., Hall, J.C. & Taghert, P.H. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99, 791–802 (1999).
Sepp, K.J., Schulte, J. & Auld, V.J. Peripheral glia direct axon guidance across the CNS/PNS transition zone. Dev. Biol. 238, 47–63 (2001).
Dimitroff, B. et al. Diet and energy-sensing inputs affect TorC1-mediated axon misrouting but not TorC2-directed synapse growth in a Drosophila model of tuberous sclerosis. PLoS One 7, e30722 (2012).
Chen, K.F., Peschel, N., Zavodska, R., Sehadova, H. & Stanewsky, R. QUASIMODO, a novel GPI-anchored zona pellucida protein involved in light input to the Drosophila circadian clock. Curr. Biol. 21, 719–729 (2011).
Levine, J.D., Funes, P., Dowse, H.B. & Hall, J.C. Signal analysis of behavioral and molecular cycles. BMC Neurosci. 3, 1–25 (2002).
Wilson, C.G. et al. Liver-specific overexpression of pancreatic-derived factor (PANDER) induces fasting hyperglycemia in mice. Endocrinology 151, 5174–5184 (2010).
Gekakis, N. et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280, 1564–1569 (1998).
Maier, B. et al. A large-scale functional RNAi screen reveals a role for CK2 in the mammalian circadian clock. Genes Dev. 23, 708–718 (2009).
Locke, J.C.W. et al. Extension of a genetic network model by iterative experimentation and mathematical analysis. Mol. Syst. Biol. 1, 2005.0013 (2005).
Klemz, R., Mashreghi, M.F., Spies, C., Volk, H.D. & Kotsch, K. Amplifying the fluorescence of bilirubin enables the real-time detection of heme oxygenase activity. Free Radic. Biol. Med. 46, 305–311 (2009).
Ripperger, J.A. & Schibler, U. Rhythmic CLOCK–BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat. Genet. 38, 369–374 (2006).
Livak, K.J. & Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Lide, D.R. CRC Handbook of Chemistry and Physics: a Ready-Reference Book of Chemical and Physical Data (CRC Press, 1995).
Gautier, L., Cope, L., Bolstad, B.M. & Irizarry, R.A. affy: analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20, 307–315 (2004).
Smyth, G.K. in Bioinformatics and Computational Biology Solutions Using R and Bioconductor (eds. Gentleman, R., Carey, V., Huber, W., Irizarry, R. & Dudoit, S.) 397–420 (Springer, 2005).
Roider, H.G., Kanhere, A., Manke, T. & Vingron, M. Predicting transcription factor affinities to DNA from a biophysical model. Bioinformatics 23, 134–141 (2007).
Flicek, P. et al. Ensembl 2012. Nucleic Acids Res. 40, D84–D90 (2012).
Acknowledgements
We thank A. Grudziecki, B. Koller and U. Ungethüm for excellent technical support. We also thank M. Rauer and H. Herzel for bioinformatics help as well as A. Zenclusen (Otto von Guericke University Magdeburg), M. Brunner (Ruprecht-Karls-University Heidelberg), S. Taketani (Insect Biomedical Research Center, Kyoto Institute of Technology) and the NIG-Fly Stock Center (Genetic Strain Research Center, National Institute of Genetics Mishima) for materials. This work was supported by the BBSRC (grant BB/J018589/1 to R.S.) and the German Research foundation (Emmy Noether grant SCHU 2546/1-1 to M.S. and SFB 618/A4 and SFB 740/D2 to A.K.).
Author information
Authors and Affiliations
Contributions
R.K., S.R., T.W., N.W., S.K., V.L., M.K., S.H., M.X. and J.A.R. performed experiments; K.J. performed bioinformatics analyses; S.L. provided the ChronoStar software; R.K., S.R., T.W., K.J., S.K., V.L., J.A.R., M.S., R.S. and A.K. designed experiments and analyzed data; R.S. and A.K. wrote the paper; and A.K. oversaw the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Heme oxygenase 1 is regulated by the circadian clock: additional data.
(a) Ho-1 mRNA levels oscillate in mouse peritoneal macrophages. These data are taken from our experiments published in Keller, M. et al. Proc. Natl. Acad. Sci. USA. 106, 21407-21412 (2009). Cells harvested every 4 h via peritoneal lavage from four C57BL/6 mice kept in constant darkness were magnetically purified for CD11b surface expression. Three individual RNA samples of each time were pooled and subjected to global gene transcription measurement by using Affymetrix microarrays. Circadian oscillation of Ho-1 transcript is significant (p < 0.005 CircWave). (b) Ho-1 mRNA levels do not oscillate in primary hepatocytes from Bmal1-/- mice. Ho-1 mRNA levels in hemin and CoPPIX treated (30 μM each) primary hepatocytes from Bmal1-/- mice. Cells were dexamethasone-synchronized and harvested after 24 hour and 40 hours, which correspond to trough and peak of Ho-1 mRNA levels in wild-type cells (see Fig. 2b). Data are normalized to Gapdh expression and presented relative to mean expression in untreated cells. Given are means ± sd of three independent samples. (c) Conserved E-box (blue) in mouse, rat and human Ho-1 promoters close the transcription start site (yellow) and start of coding region (red). (d) Ho-1 mRNA levels are reduced in unsynchronized primary hepatocytes from Bmal1-/- mice compared to hepatocytes from wild-type littermates (wt). Data are normalized to Gapdh expression and presented relative to mean expression in wild-type cells. Given is mean ± sd of three independent samples.
Supplementary Figure 2 The heme-degradation product CO modulates circadian transcription: additional data.
Upper left: Transcript levels of Rev-Erbα and Dbp in CO or N2 (6%) treated primary fibroblasts of Ho-1-/- mice or wild-type littermates harvested 24 hours after dexamethasone synchronization. Other panels: Transcript levels of Dbp in embryonic fibroblasts from Ho-1-/- mice (Ho1KO) or wild-type littermates (Ho1WT) 24 hours after dexamethasone synchronization, which were treated for 1 hour (or 3 x 1 hour; lower right) with 100 μM CO-releasing molecules (CORM) or inactive control molecules (iCORM) before harvesting. Data are normalized to Gapdh expression and presented relative to mean expression in wild-type control cells. Given is mean ± sem of three independent samples or – upper left – mean (bars) of two independent samples (small symbols).
Supplementary Figure 3 CO modulates clock-gene expression at the transcriptional level.
(a) Clock gene mRNA and pre-mRNA levels of primary fibroblasts of Ho-1-/- mice (KO) or wild-type littermates (WT) harvested 24 hours after dexamethasone synchronization. Data are normalized to Gapdh expression and presented relative to mean expression in wild-type control cells. Given is mean ± sd of three independent samples. (b) Pre-mRNA levels of Dbp in embryonic fibroblasts from Ho-1-/- mice (KO) or wild-type littermates (WT) 24 hours after dexamethasone synchronization, which were treated for 1 hour with 100 μM CO-releasing molecules (CORM) or inactive control molecules (iCORM) before harvesting. Data are normalized to Gapdh expression and presented relative to mean expression in wild-type control cells. Given is mean ± sd of three independent samples. (c) CO does not acutely alter BMAL1 protein level. BMAL1 protein levels in U2-OS cells 24 hours after dexamethasone synchronization, which were treated for 1 hour with 100 μM CO-releasing molecules (CORM) or inactive control molecules (iCORM) before harvesting. Shown are four independent samples.
Supplementary Figure 4 Ho-1 knockout alone has no effect on circadian dynamics, probably because of residual activity from HO-2.
(a) Circadian oscillation dynamics of synchronized primary fibroblasts of Ho-1-/- mice (Ho1KO) or wild-type littermates (Ho1WT) lentivirally transduced with a Bmal1 promoter-luciferase reporter construct. Shown are two representative examples of raw data (upper panel) and detrended (lower panel) time-series for each genotype. Note, the absolute light levels slightly decrease upon Ho-1 knockout consistent with the overall slightly lower Bmal1 transcript levels (compare Fig. 3a). (b) Heme oxygenase activity in fibroblasts with specific depletion/knockout of heme oxygenase isoforms. Lysates of primary fibroblasts of indicated Ho-1 genotype with or without additional RNAi-mediated depletion of Ho-2 were analyzed for total heme oxygenase activity.
Supplementary Figure 5 Heme oxygenase–derived CO is essential for normal circadian dynamics in mammalian cells: additional data.
(a) Circadian dynamics of synchronized primary fibroblasts from Ho-1-/- mice lentivirally transduced with (i) shRNA constructs targeting Ho-2 or a non-silencing (ns) control and (ii) a Bmal1 promoter-luciferase reporter construct. Shown are representative examples of raw data time-series. For detrended time series and period quantification see Fig. 5a. Note, that the absolute light levels decrease upon Ho‑2 knockdown consistent with the overall increase in Rev-Erbα levels and thus putatively lower Bmal1 transcript levels (compare Fig. 5b). (b) CO partly rescues the long period oscillations in heme oxygenase depleted cells. Circadian oscillation dynamics of synchronized primary fibroblasts from Ho-1-/- mice lentivirally transduced with (i) shRNA constructs targeting Ho-2 and (ii) a Bmal1 promoter-luciferase reporter construct. Cells were continuously treated with 6% CO or N2. Shown are representative examples of raw data time-series. For detrended time series see Fig. 5c. (c) Exogenous CO treatment has no effect on oscillation dynamics of wild-type cells. Circadian oscillation dynamics of synchronized primary fibroblasts from wild-type mice lentivirally transduced with a Bmal1 promoter-luciferase reporter construct, which were continuously treated with 6% CO or N2. Shown is a representative example of raw data (upper panel) and detrended time-series (lower panel). Note, since CO can only shorten the circadian period in Ho-depleted cells (Fig. 5c) but not in wild-type cells, period lengthening in Ho-depleted cells is very likely specifically due to the lack of endogenous CO rather than to other functions of heme oxygenases.
Supplementary Figure 6 Heme oxygenases modulate metabolic gene expression in primary hepatocytes at the transcriptional level.
Pre-mRNA levels of Pck1, G6pc, Lpl and Cyp7a1 in primary hepatocytes (24 hours after dexamethasone synchronization) of Ho‑1-/- or wild-type littermate mice with or without additional Ho-2 depletion by RNAi. Data are normalized to Gapdh expression and presented relative to mean expression in wild-type cells transduced with the non-silencing control. Shown are mean levels (bars) of two independent samples (small symbols).
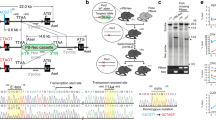
Supplementary Figure 7 Heme oxygenase (dHo) is essential for normal daily activity patterns in Drosophila: additional data.
(a) Mean locomotor activity of Drosophila males analysed in 12 hr light: 12 hr dark conditions for 5-8 days. A different dHo-RNAi construct inserted either on chromosome 3 (UAS-dHo-RNAi:R1) or chromosome 2 (UAS-dHo-RNAi:R3) was either driven in all clock cells: timeless-gal4 (tim >); PDF-positive clock neurons: Pdf-gal4 (Pdf >); all neurons: elav-gal4 (elav >), or all glia cells: repo-gal4 (repo>). Note the phase advance caused by dHo knockdown in clock cells and neurons, whereas glia-specific knockdown or ‘driver-only’ controls results in normal behaviour. At least 16 flies were tested for each genotype. Bars show mean activity within in 30 min; dark bars: ‘lights-off’, white bars: ‘lights on’, dots indicate sem. (b) Real-time luciferase recordings of flies expressing different period-luciferase fusion genes. Male flies were recorded in LD and DD as indicated by the bars below the plots (white and black bars indicate ‘lights-on’ and ‘lights-off’ respectively). Left: Flies expressing luciferase under control of the period promoter in all clock cells (including peripheral clocks) encoded by the plo transgene29: grey circles—tim-gal4:27/+;plo:86-6/UAS-dHo-RNAi:21-1 (n=16); black circles—tim-gal4:27/UAS-GFP;plo:86:6/+ (n=12). Middle: Flies expressing a PERIOD-LUCIFERASE fusion protein in all clock cells (including peripheral clocks) encoded by the XLG-luc transgene30: grey circles—tim-gal4:27/+;XLG-luc/UAS-dHo-RNAi:21-1 (n=8); black circles—tim-gal4:27/UAS-GFP;XLG-luc/+ (n=8); open circles—tim-gal4:27/UAS-attP-51C;XLG-luc/+ (n=4). Note that in the left and middle panels no significant differences are observable between dHo-RNAi and control flies. Similar results were obtained in two independent experiments including the dHoRNAi:21-8 line. Right: Flies expressing a PERIOD-LUCIFERASE fusion protein in dorsal clock neurons encoded by the promoter-less 8.0-luc transgene30. Exact genotypes: grey circles—8.0-luc/+;tim-gal4:67/UAS-dHo-RNAi:21-8 (n=8); black circles—8.0-luc/UAS-GFP;tim-gal4:67/+ (n=10). Note the higher peak levels in LD and DD, and the increased amplitude of PER-LUC oscillations in dHo-RNAi flies.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Table 2 and Supplementary Notes 1 and 2 (PDF 1412 kb)
Supplementary Table 1
Heme oxygenase depletion globally alters clock-controlled transcription in hepatocytes (XLSX 392 kb)
Rights and permissions
About this article
Cite this article
Klemz, R., Reischl, S., Wallach, T. et al. Reciprocal regulation of carbon monoxide metabolism and the circadian clock. Nat Struct Mol Biol 24, 15–22 (2017). https://doi.org/10.1038/nsmb.3331
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3331
This article is cited by
-
Circadian dependency of microglial heme oxygenase-1 expression and inflammation determine neuronal injury in hemorrhagic stroke
Journal of Inflammation (2023)
-
ASIC1a affects hypothalamic signaling and regulates the daily rhythm of body temperature in mice
Communications Biology (2023)
-
Capture of carbon monoxide using a heme protein model: from biomimetic chemistry of heme proteins to physiological and therapeutic applications
Polymer Journal (2022)
-
Six degrees head-down tilt bed rest caused low-grade hemolysis: a prospective randomized clinical trial
npj Microgravity (2021)
-
Carbon monoxide: a critical physiological regulator sensitive to light
Translational Psychiatry (2020)