Abstract
Heat-shock transcription factor 1 (HSF1) has a central role in mediating the protective response to protein conformational stresses in eukaryotes. HSF1 consists of an N-terminal DNA-binding domain (DBD), a coiled-coil oligomerization domain, a regulatory domain and a transactivation domain. Upon stress, HSF1 trimerizes via its coiled-coil domain and binds to the promoters of heat shock protein–encoding genes. Here, we present cocrystal structures of the human HSF1 DBD in complex with cognate DNA. A comparative analysis of the HSF1 paralog Skn7 from Chaetomium thermophilum showed that single amino acid changes in the DBD can switch DNA binding specificity, thus revealing the structural basis for the interaction of HSF1 with cognate DNA. We used a crystal structure of the coiled-coil domain of C. thermophilum Skn7 to develop a model of the active human HSF1 trimer in which HSF1 embraces the heat-shock-element DNA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Balch, W.E., Morimoto, R.I., Dillin, A. & Kelly, J.W. Adapting proteostasis for disease intervention. Science 319, 916–919 (2008).
Kim, Y.E., Hipp, M.S., Bracher, A., Hayer-Hartl, M. & Hartl, F.U. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 82, 323–355 (2013).
Åkerfelt, M., Morimoto, R.I. & Sistonen, L. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 11, 545–555 (2010).
Vabulas, R.M., Raychaudhuri, S., Hayer-Hartl, M. & Hartl, F.U. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb. Perspect. Biol. 2, a004390 (2010).
Anckar, J. & Sistonen, L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu. Rev. Biochem. 80, 1089–1115 (2011).
Morimoto, R.I. The heat shock response: systems biology of proteotoxic stress in aging and disease. Cold Spring Harb. Symp. Quant. Biol. 76, 91–99 (2011).
Pelham, H.R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell 30, 517–528 (1982).
Amin, J., Ananthan, J. & Voellmy, R. Key features of heat shock regulatory elements. Mol. Cell. Biol. 8, 3761–3769 (1988).
Xiao, H. & Lis, J.T. Germline transformation used to define key features of heat-shock response elements. Science 239, 1139–1142 (1988).
Wu, C. Heat shock transcription factors: structure and regulation. Annu. Rev. Cell Dev. Biol. 11, 441–469 (1995).
Pattaramanon, N., Sangha, N. & Gafni, A. The carboxy-terminal domain of heat-shock factor 1 is largely unfolded but can be induced to collapse into a compact, partially structured state. Biochemistry 46, 3405–3415 (2007).
Harrison, C.J., Bohm, A.A. & Nelson, H.C. Crystal structure of the DNA binding domain of the heat shock transcription factor. Science 263, 224–227 (1994).
Vuister, G.W. et al. Solution structure of the DNA-binding domain of Drosophila heat shock transcription factor. Nat. Struct. Biol. 1, 605–614 (1994).
Liu, G. et al. Solution NMR structure of heat shock factor protein 1 DNA binding domain from Homo sapiens, Northeast Structural Genomics Consortium Target HR3023C (2011).
Littlefield, O. & Nelson, H.C. A new use for the 'wing' of the 'winged' helix-turn-helix motif in the HSF–DNA cocrystal. Nat. Struct. Biol. 6, 464–470 (1999).
Baler, R., Dahl, G. & Voellmy, R. Activation of human heat shock genes is accompanied by oligomerization, modification, and rapid translocation of heat shock transcription factor HSF1. Mol. Cell. Biol. 13, 2486–2496 (1993).
Sarge, K.D., Murphy, S.P. & Morimoto, R.I. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol. Cell. Biol. 13, 1392–1407 (1993).
Xiao, X. et al. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 18, 5943–5952 (1999).
Mendillo, M.L. et al. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell 150, 549–562 (2012).
Santagata, S. et al. Tight coordination of protein translation and HSF1 activation supports the anabolic malignant state. Science 341, 1238303 (2013).
Dai, C., Whitesell, L., Rogers, A.B. & Lindquist, S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 130, 1005–1018 (2007).
Scherz-Shouval, R. et al. The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell 158, 564–578 (2014).
Mercier, P.A., Winegarden, N.A. & Westwood, J.T. Human heat shock factor 1 is predominantly a nuclear protein before and after heat stress. J. Cell Sci. 112, 2765–2774 (1999).
Rabindran, S.K., Haroun, R.I., Clos, J., Wisniewski, J. & Wu, C. Regulation of heat shock factor trimer formation: role of a conserved leucine zipper. Science 259, 230–234 (1993).
Zuo, J., Baler, R., Dahl, G. & Voellmy, R. Activation of the DNA-binding ability of human heat shock transcription factor 1 may involve the transition from an intramolecular to an intermolecular triple-stranded coiled-coil structure. Mol. Cell. Biol. 14, 7557–7568 (1994).
Neef, D.W., Jaeger, A.M. & Thiele, D.J. Genetic selection for constitutively trimerized human HSF1 mutants identifies a role for coiled-coil motifs in DNA binding. G3 (Bethesda) 3, 1315–1324 (2013).
Abravaya, K., Myers, M.P., Murphy, S.P. & Morimoto, R.I. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev. 6, 1153–1164 (1992).
Nadeau, K., Das, A. & Walsh, C.T. Hsp90 chaperonins possess ATPase activity and bind heat shock transcription factors and peptidyl prolyl isomerases. J. Biol. Chem. 268, 1479–1487 (1993).
Zou, J., Guo, Y., Guettouche, T., Smith, D.F. & Voellmy, R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94, 471–480 (1998).
Neef, D.W. et al. A direct regulatory interaction between chaperonin TRiC and stress-responsive transcription factor HSF1. Cell Rep. 9, 955–966 (2014).
Shi, Y., Mosser, D.D. & Morimoto, R.I. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 12, 654–666 (1998).
Morimoto, R.I. Dynamic remodeling of transcription complexes by molecular chaperones. Cell 110, 281–284 (2002).
Larson, J.S., Schuetz, T.J. & Kingston, R.E. In vitro activation of purified human heat shock factor by heat. Biochemistry 34, 1902–1911 (1995).
Kline, M.P. & Morimoto, R.I. Repression of the heat shock factor 1 transcriptional activation domain is modulated by constitutive phosphorylation. Mol. Cell. Biol. 17, 2107–2115 (1997).
Hietakangas, V. et al. Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol. Cell. Biol. 23, 2953–2968 (2003).
Westerheide, S.D., Anckar, J., Stevens, S.M. Jr., Sistonen, L. & Morimoto, R.I. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 323, 1063–1066 (2009).
Raychaudhuri, S. et al. Interplay of acetyltransferase EP300 and the proteasome system in regulating heat shock transcription factor 1. Cell 156, 975–985 (2014).
Grady, D.L. et al. Highly conserved repetitive DNA sequences are present at human centromeres. Proc. Natl. Acad. Sci. USA 89, 1695–1699 (1992).
Denegri, M. et al. Human chromosomes 9, 12, and 15 contain the nucleation sites of stress-induced nuclear bodies. Mol. Biol. Cell 13, 2069–2079 (2002).
Jolly, C. et al. In vivo binding of active heat shock transcription factor 1 to human chromosome 9 heterochromatin during stress. J. Cell Biol. 156, 775–781 (2002).
Jolly, C. et al. Stress-induced transcription of satellite III repeats. J. Cell Biol. 164, 25–33 (2004).
Metz, A., Soret, J., Vourc'h, C., Tazi, J. & Jolly, C. A key role for stress-induced satellite III transcripts in the relocalization of splicing factors into nuclear stress granules. J. Cell Sci. 117, 4551–4558 (2004).
Flick, K.E., Gonzalez, L. Jr., Harrison, C.J. & Nelson, H.C. Yeast heat shock transcription factor contains a flexible linker between the DNA-binding and trimerization domains: implications for DNA binding by trimeric proteins. J. Biol. Chem. 269, 12475–12481 (1994).
Zelin, E., Zhang, Y., Toogun, O.A., Zhong, S. & Freeman, B.C. The p23 molecular chaperone and GCN5 acetylase jointly modulate protein-DNA dynamics and open chromatin status. Mol. Cell 48, 459–470 (2012).
Ahn, S.G. & Thiele, D.J. Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev. 17, 516–528 (2003).
Lu, M. et al. Two distinct disulfide bonds formed in human heat shock transcription factor 1 act in opposition to regulate its DNA binding activity. Biochemistry 47, 6007–6015 (2008).
Xiao, H., Perisic, O. & Lis, J.T. Cooperative binding of Drosophila heat shock factor to arrays of a conserved 5 bp unit. Cell 64, 585–593 (1991).
Sorger, P.K. & Pelham, H.R. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell 54, 855–864 (1988).
Wiederrecht, G., Seto, D. & Parker, C.S. Isolation of the gene encoding the S. cerevisiae heat shock transcription factor. Cell 54, 841–853 (1988).
Fassler, J.S. & West, A.H. Fungal Skn7 stress responses and their relationship to virulence. Eukaryot. Cell 10, 156–167 (2011).
Li, S. et al. The yeast histidine protein kinase, Sln1p, mediates phosphotransfer to two response regulators, Ssk1p and Skn7p. EMBO J. 17, 6952–6962 (1998).
Morgan, B.A. et al. The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae. EMBO J. 16, 1035–1044 (1997).
Li, S. et al. The eukaryotic two-component histidine kinase Sln1p regulates OCH1 via the transcription factor, Skn7p. Mol. Biol. Cell 13, 412–424 (2002).
Peteranderl, R. & Nelson, H.C. Trimerization of the heat shock transcription factor by a triple-stranded alpha-helical coiled-coil. Biochemistry 31, 12272–12276 (1992).
Sandqvist, A. et al. Heterotrimerization of heat-shock factors 1 and 2 provides a transcriptional switch in response to distinct stimuli. Mol. Biol. Cell 20, 1340–1347 (2009).
Neef, D.W., Jaeger, A.M. & Thiele, D.J. Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat. Rev. Drug Discov. 10, 930–944 (2011).
Alarcon, S.V. et al. Tumor-intrinsic and tumor-extrinsic factors impacting hsp90- targeted therapy. Curr. Mol. Med. 12, 1125–1141 (2012).
Calamini, B. & Morimoto, R.I. Protein homeostasis as a therapeutic target for diseases of protein conformation. Curr. Top. Med. Chem. 12, 2623–2640 (2012).
Treisman, J., Gönczy, P., Vashishtha, M., Harris, E. & Desplan, C. A single amino acid can determine the DNA binding specificity of homeodomain proteins. Cell 59, 553–562 (1989).
Tucker-Kellogg, L. et al. Engrailed (Gln50Lys) homeodomain-DNA complex at 1.9 Å resolution: structural basis for enhanced affinity and altered specificity. Structure 5, 1047–1054 (1997).
Catanzariti, A.M., Soboleva, T.A., Jans, D.A., Board, P.G. & Baker, R.T. An efficient system for high-level expression and easy purification of authentic recombinant proteins. Protein Sci. 13, 1331–1339 (2004).
Jaeger, A.M., Makley, L.N., Gestwicki, J.E. & Thiele, D.J. Genomic heat shock element sequences drive cooperative human heat shock factor 1 DNA binding and selectivity. J. Biol. Chem. 289, 30459–30469 (2014).
Wyatt, P.J. Light scattering and the absolute characterization of macromolecules. Anal. Chim. Acta 272, 1–40 (1993).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D Biol. Crystallogr. 62, 72–82 (2006).
Evans, P.R. Scala. Joint CCP4 ESF-EACBM Newsl. 33, 22–24 (1997).
Evans, P.R. & Murshudov, G.N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 69, 1204–1214 (2013).
French, G. & Wilson, K. On the treatment of negative intensity observations. Acta Crystallogr. A 34, 517–525 (1978).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Pape, T. & Schneider, T.R. HKL2MAP: a graphical user interface for phasing with SHELX programs. J. Appl. Crystallogr. 37, 843–844 (2004).
Sheldrick, G.M. Experimental phasing with SHELXC/D/E: combining chain tracing with density modification. Acta Crystallogr. D Biol. Crystallogr. 66, 479–485 (2010).
Langer, G., Cohen, S.X., Lamzin, V.S. & Perrakis, A. Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nat. Protoc. 3, 1171–1179 (2008).
Vagin, A.A. & Isupov, M.N. Spherically averaged phased translation function and its application to the search for molecules and fragments in electron-density maps. Acta Crystallogr. D Biol. Crystallogr. 57, 1451–1456 (2001).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Kleywegt, G.T. & Jones, T.A. A super position. Joint CCP4 ESF-EACBM Newsl. 31, 9–14 (1994).
Sali, A. & Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Fiser, A., Do, R.K. & Sali, A. Modeling of loops in protein structures. Protein Sci. 9, 1753–1773 (2000).
Fiser, A. & Sali, A. ModLoop: automated modeling of loops in protein structures. Bioinformatics 19, 2500–2501 (2003).
Pettersen, E.F. et al. UCSF Chimera: a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Gouet, P., Courcelle, E., Stuart, D.I. & Métoz, F. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15, 305–308 (1999).
Acknowledgements
We thank R. Körner for MS analysis, R. Lange and A. Jungclaus for assistance with protein purification, and the staff at the Core and Crystallization Facilities at the Max Planck Institute of Biochemistry and at the European Synchrotron Radiation Facility (ESRF) in Grenoble, France, for their excellent services. J.V. is supported by a Rudolf Haas Fellowship from the Jung Foundation for Science and Research.
Author information
Authors and Affiliations
Contributions
T.N. performed the biochemical and functional analysis and obtained the crystals. A.B. and T.N. solved the crystal structures, J.V. performed the cell culture experiments, and M.H.-H. collected SEC-MALS measurements. A.B. and F.U.H. supervised the experimental design and data interpretation. All authors contributed to experimental design, data analyses and manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 The DNA-binding domain of HsHSF1 (HsDBD).
(a-c) Superposition of the HsDBD structure in the HsDBD–HSE complex with the DBDs in the HsDBD–SatIII complex (a), with the NMR structure of the free HsDBD (PDB 2LDU, Liu, G. et al., 2011) (b) and with the KlDBD–HSE complex (PDB 3HTS, Littlefield, O. & Nelson, H.C. Nat. Struct. Biol. 6, 464-470, 1999) (c). Cα traces are shown. (d) Interface between the C-terminal 20 residues and the main body of the HsDBD. The protein backbone of HsDBD from the HsDBD–SatIII complex is shown in ribbon representation; residues 13-100 are additionally represented as molecular surface. The hydrophobic interface residues in the C-terminal segment are highlighted in stick representation. The hydrogen bond contact to helix α3 is shown as a dotted line.
Supplementary Figure 2 DBD-DBD interactions in HSF1–DNA complexes.
(a, b) The KlDBD–HSE complex crystal structure (pdb 3HTS) viewed along the two-fold symmetry axis (a), with the molecular contacts in the DBD–DBD interface highlighted in (b). Protein is shown in ribbon representation; DNA is shown in surface representation. Prominent sidechains are shown in stick representation; hydrogen bonds are represented by dotted lines. (c) Interactions between two asymmetric units along the DNA stacks in the crystal lattice of the HsDBD–SatIII complex. The duplexes are rotated out of register. Furthermore, two additional nucleotides were included into the DNA, shifting the DBDs ~6.8 Å further apart. (d, e) Potential interaction site of the wing domain in head-to-tail contacts. A view along the DNA is shown. A hydrophobic groove is situated between helices 2 and 3 (indicated by an arrow). Residues lining the groove are highlighted in stick representation. Panel e shows a surface representation with positive and negative charges is blue and red, respectively. Hydrophobic sidechains are shown in yellow.
Supplementary Figure 3 Sequence alignment of the conserved segment of HsHSF1, CtHSF1 and CtSkn7.
Secondary structure elements are indicated above and below the sequences. Similar residues are shown in red and identical residues in white using bold lettering on red background. Blue frames indicate homologous regions. The downward arrowhead designates the residue critical for Skn7 DNA-binding properties. Asterisks denote acetylation sites in HsHSF1; red ovals designate putative hydrophobic layer residues in the HsHSF1 coiled-coil.
Supplementary Figure 4 Interaction of CtSkn7 with DNA.
(a) Superposition of the DBDs from HsDBD–HSE and CtDBD–HSE complexes. (b) Superposition of the DBD pairs from CtDBD–HSE and CtDBD–SSRE structures. (c) Sequence alignment of representative Skn7 and HSF1 sequences, showing the systematic sequence divergence at residue 100 (CtSkn7 numbering). (d) Close-up of the interactions of CtDBD with the GGC triplet in SSRE. Sidechains of conserved residues are shown in stick representation. Hydrogen bonds are represented by dotted lines. The C-terminal tail of the DBD was omitted for clarity. (e, f) Omit density for the base-specific interactions in the CtDBD–SSRE complex. Panels e and f show the interactions of helix 3 with the GCC and GGC motifs, respectively (same orientation as in Fig. 4c). For calculating omit density, CtDBD residues 95-101 were deleted from the model, 0.2 Å random shifts applied to the coordinates with PDBSET and B-factors set to 20.00 Å2 to reduce model bias. These modified models were re-refined with Refmac5. Weighted 2Fo-Fc density maps at 1.0 sigma are shown for the missing segments, superposed on the complete model. Water atoms and the C-terminal tail residues were deleted for clarity. (g) Close-up of the interactions of CtDBD with the GAA triplet in HSE.
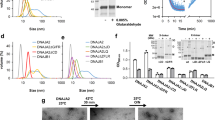
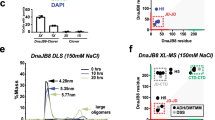
Supplementary Figure 5 Biophysical characterization of CtSkn7 and the chimeric HsHSF1(CtHR-A/B) protein.
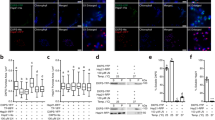
(a, b) Limited proteolysis of CtSkn7(40–220) in absence (a) and presence (b) of HSE DNA duplexes. CtSkn7(40–220) at 11 μM was treated with increasing amounts of proteinase K (PK) (0, 0.05, 0.15, 0.45, 1.35, 4.05, 12.15 and 36.45 μg ml-1) for 1 h on ice. The reaction was terminated by addition of 4 mM phenylmethylsulfonyl fluoride. The products were analyzed by SDS-PAGE and Coomassie staining. (c, d) SEC-MALS analysis of CtSkn7(160–209) (c) and HsHSF1(CtHR-A/B) (d). Data show measurements of ~100 μg protein. The calculated molar mass is indicated. The theoretical molar masses of CtSkn7(160–209) dimers and trimers are ~11.4 and ~17.1 kDa, respectively. The average observed mass of ~14.5 kDa is consistent with a ~1:1 mixture of dimer and trimer. The theoretical mass for HsHSF1(CtHR-A/B) trimer is ~171.8 kDa. (e, f) Cellular locations of HsHSF1-V (e) and HsHSF1(CtHR-A/B)-V (f) in HeLa cells in absence and presence of heat shock (HS) treatment. Nuclei are stained with DAPI.
Supplementary Figure 6 Structural model for HsHSF1(13–182) bound to SatIII repeats.
Perpendicular views are shown. The C-termini point alternatingly in opposite directions; only every second HsHSF1 chain can contribute to a trimeric complex. The three HsHSF1 chains in one complex are indicated in blue, yellow and beige. The DNA is shown in stick representation. The linkers were modelled with Modeller as implemented in the ModLoop server. The helical bundle is a homology model based on the CtSkn7(160–209) trimer structure.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Table 1 (PDF 1648 kb)
Rights and permissions
About this article
Cite this article
Neudegger, T., Verghese, J., Hayer-Hartl, M. et al. Structure of human heat-shock transcription factor 1 in complex with DNA. Nat Struct Mol Biol 23, 140–146 (2016). https://doi.org/10.1038/nsmb.3149
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3149
This article is cited by
-
Alternative Splicing Reveals Acute Stress Response of Litopenaeus vannamei at High Alkalinity
Marine Biotechnology (2024)
-
Genomic and epigenomic determinants of heat stress-induced transcriptional memory in Arabidopsis
Genome Biology (2023)
-
Missense mutation of a class B heat shock factor is responsible for the tomato bushy root-2 phenotype
Molecular Horticulture (2022)
-
RNA-seq Analysis Reveals Alternative Splicing Under Heat Stress in Rainbow Trout (Oncorhynchus mykiss)
Marine Biotechnology (2022)
-
Transcriptional regulation of small heat shock protein genes by heat shock factor 1 (HSF1) in Liriomyza trifolii under heat stress
Cell Stress and Chaperones (2021)