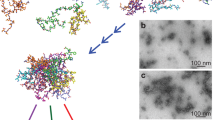
Abstract
Small heat-shock proteins, including αB-crystallin (αB), play an important part in protein homeostasis, because their ATP-independent chaperone activity inhibits uncontrolled protein aggregation. Mechanistic details of human αB, particularly in its client-bound state, have been elusive so far, owing to the high molecular weight and the heterogeneity of these complexes. Here we provide structural insights into this highly dynamic assembly and show, by using state-of-the-art NMR spectroscopy, that the αB complex is assembled from asymmetric building blocks. Interaction studies demonstrated that the fibril-forming Alzheimer's disease Aβ1–40 peptide preferentially binds to a hydrophobic edge of the central β-sandwich of αB. In contrast, the amorphously aggregating client lysozyme is captured by the partially disordered N-terminal domain of αB. We suggest that αB uses its inherent structural plasticity to expose distinct binding interfaces and thus interact with a wide range of structurally variable clients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Haslbeck, M. & Vierling, E. A first line of stress defense: small heat shock proteins and their function in protein homeostasis. J. Mol. Biol. 427, 1537–1548 (2015).
Horwitz, J. Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA 89, 10449–10453 (1992).
Horwitz, J. Alpha crystallin: the quest for a homogeneous quaternary structure. Exp. Eye Res. 88, 190–194 (2009).
Aquilina, J.A., Benesch, J.L.P., Bateman, O.A., Slingsby, C. & Robinson, C.V. Polydispersity of a mammalian chaperone: mass spectrometry reveals the population of oligomers in αB-crystallin. Proc. Natl. Acad. Sci. USA 100, 10611–10616 (2003).
Jehle, S. et al. Solid-state NMR and SAXS studies provide a structural basis for the activation of αB-crystallin oligomers. Nat. Struct. Mol. Biol. 17, 1037–1042 (2010).
Laganowsky, A. et al. Crystal structures of truncated alphaA and alphaB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Sci. 19, 1031–1043 (2010).
Delbecq, S.P., Jehle, S. & Klevit, R. Binding determinants of the small heat shock protein, αB-crystallin: recognition of the 'IxI' motif. EMBO J. 31, 4587–4594 (2012).
Baldwin, A.J. et al. Quaternary dynamics of αB-crystallin as a direct consequence of localised tertiary fluctuations in the C-terminus. J. Mol. Biol. 413, 310–320 (2011).
Baldwin, A.J., Lioe, H., Robinson, C.V., Kay, L.E. & Benesch, J.L.P. αB-crystallin polydispersity is a consequence of unbiased quaternary dynamics. J. Mol. Biol. 413, 297–309 (2011).
Delbecq, S.P. & Klevit, R.E. One size doesn't fit all: the oligomeric states of αB crystallin. FEBS Lett. 587, 1073–1080 (2013).
Jehle, S. et al. alphaB-crystallin: a hybrid solid-state/solution-state NMR investigation reveals structural aspects of the heterogeneous oligomer. J. Mol. Biol. 385, 1481–1497 (2009).
Mainz, A. et al. Structural and mechanistic implications of metal binding in the small heat-shock protein αB-crystallin. J. Biol. Chem. 287, 1128–1138 (2012).
Bagnéris, C. et al. Crystal structures of alpha-crystallin domain dimers of alphaB-crystallin and Hsp20. J. Mol. Biol. 392, 1242–1252 (2009).
Braun, N. et al. Multiple molecular architectures of the eye lens chaperone αB-crystallin elucidated by a triple hybrid approach. Proc. Natl. Acad. Sci. USA 108, 20491–20496 (2011).
Clark, A.R., Naylor, C.E., Bagnéris, C., Keep, N.H. & Slingsby, C. Crystal structure of R120G disease mutant of human αB-crystallin domain dimer shows closure of a groove. J. Mol. Biol. 408, 118–134 (2011).
Jehle, S. et al. N-terminal domain of αB-crystallin provides a conformational switch for multimerization and structural heterogeneity. Proc. Natl. Acad. Sci. USA 108, 6409–6414 (2011).
Baldwin, A.J. et al. Probing dynamic conformations of the high-molecular-weight αB-crystallin heat shock protein ensemble by NMR spectroscopy. J. Am. Chem. Soc. 134, 15343–15350 (2012).
Baldwin, A.J. et al. The polydispersity of αB-crystallin is rationalized by an interconverting polyhedral architecture. Structure 19, 1855–1863 (2011).
Ghosh, J.G., Shenoy, A.K. Jr. & Clark, J.I. Interactions between important regulatory proteins and human alphaB crystallin. Biochemistry 46, 6308–6317 (2007).
Rekas, A. et al. Interaction of the molecular chaperone alphaB-crystallin with alpha-synuclein: effects on amyloid fibril formation and chaperone activity. J. Mol. Biol. 340, 1167–1183 (2004).
Waudby, C.A. et al. The interaction of alphaB-crystallin with mature alpha-synuclein amyloid fibrils inhibits their elongation. Biophys. J. 98, 843–851 (2010).
Shammas, S.L. et al. Binding of the molecular chaperone αB-crystallin to Aβ amyloid fibrils inhibits fibril elongation. Biophys. J. 101, 1681–1689 (2011).
Houck, S.A., Landsbury, A., Clark, J.I. & Quinlan, R.A. Multiple sites in αB-crystallin modulate its interactions with desmin filaments assembled in vitro. PLoS ONE 6, e25859 (2011).
Regini, J.W. et al. The interaction of unfolding α-lactalbumin and malate dehydrogenase with the molecular chaperone αB-crystallin: a light and X-ray scattering investigation. Mol. Vis. 16, 2446–2456 (2010).
Treweek, T.M., Meehan, S., Ecroyd, H. & Carver, J.A. Small heat-shock proteins: important players in regulating cellular proteostasis. Cell. Mol. Life Sci. 72, 429–451 (2015).
Sandilands, A. et al. Altered aggregation properties of mutant gamma-crystallins cause inherited cataract. EMBO J. 21, 6005–6014 (2002).
Sun, Y. & MacRae, T.H. The small heat shock proteins and their role in human disease. FEBS J. 272, 2613–2627 (2005).
Raman, B. et al. AlphaB-crystallin, a small heat-shock protein, prevents the amyloid fibril growth of an amyloid beta-peptide and beta2-microglobulin. Biochem. J. 392, 573–581 (2005).
Kulig, M. & Ecroyd, H. The small heat-shock protein αB-crystallin uses different mechanisms of chaperone action to prevent the amorphous versus fibrillar aggregation of α-lactalbumin. Biochem. J. 448, 343–352 (2012).
Ghosh, J.G., Estrada, M.R. & Clark, J.I. Interactive domains for chaperone activity in the small heat shock protein, human alphaB crystallin. Biochemistry 44, 14854–14869 (2005).
Ghosh, J.G., Estrada, M.R., Houck, S.A. & Clark, J.I. The function of the beta3 interactive domain in the small heat shock protein and molecular chaperone, human alphaB crystallin. Cell Stress Chaperones 11, 187–197 (2006).
Ghosh, J.G., Estrada, M.R. & Clark, J.I. Structure-based analysis of the beta8 interactive sequence of human alphaB crystallin. Biochemistry 45, 9878–9886 (2006).
Bhattacharyya, J., Padmanabha Udupa, E.G., Wang, J. & Sharma, K.K. Mini-alphaB-crystallin: a functional element of alphaB-crystallin with chaperone-like activity. Biochemistry 45, 3069–3076 (2006).
Mainz, A., Jehle, S., van Rossum, B.J., Oschkinat, H. & Reif, B. Large protein complexes with extreme rotational correlation times investigated in solution by magic-angle-spinning NMR spectroscopy. J. Am. Chem. Soc. 131, 15968–15969 (2009).
Mainz, A. et al. NMR spectroscopy of soluble protein complexes at one mega-dalton and beyond. Angew. Chem. Int. Ed. Engl. 52, 8746–8751 (2013).
Carver, J.A., Aquilina, J.A., Truscott, R.J. & Ralston, G.B. Identification by 1H NMR spectroscopy of flexible C-terminal extensions in bovine lens alpha-crystallin. FEBS Lett. 311, 143–149 (1992).
Treweek, T.M., Rekas, A., Walker, M.J. & Carver, J.A. A quantitative NMR spectroscopic examination of the flexibility of the C-terminal extensions of the molecular chaperones, αA- and αB-crystallin. Exp. Eye Res. 91, 691–699 (2010).
Chimon, S. et al. Evidence of fibril-like β-sheet structures in a neurotoxic amyloid intermediate of Alzheimer's β-amyloid. Nat. Struct. Mol. Biol. 14, 1157–1164 (2007).
Chimon, S. & Ishii, Y. Capturing intermediate structures of Alzheimer's beta-amyloid, Aβ(1–40), by solid-state NMR spectroscopy. J. Am. Chem. Soc. 127, 13472–13473 (2005).
Narayanan, S., Kamps, B., Boelens, W.C. & Reif, B. αB-crystallin competes with Alzheimer's disease β-amyloid peptide for peptide–peptide interactions and induces oxidation of Aβ-Met35. FEBS Lett. 580, 5941–5946 (2006).
Schütz, A.K. et al. Atomic-resolution three-dimensional structure of amyloid β fibrils bearing the Osaka mutation. Angew. Chem. Int. Ed. Engl. 54, 331–335 (2015).
Petkova, A.T. et al. A structural model for Alzheimer's β-amyloid fibrils based on experimental constraints from solid state NMR. Proc. Natl. Acad. Sci. USA 99, 16742–16747 (2002).
Paravastu, A.K., Leapman, R.D., Yau, W.-M. & Tycko, R. Molecular structural basis for polymorphism in Alzheimer's beta-amyloid fibrils. Proc. Natl. Acad. Sci. USA 105, 18349–18354 (2008).
Hochberg, G.K.A. & Benesch, J.L.P. Dynamical structure of αB-crystallin. Prog. Biophys. Mol. Biol. 115, 11–20 (2014).
Hilton, G.R. et al. C-terminal interactions mediate the quaternary dynamics of αB-crystallin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20110405 (2013).
Bertini, I. et al. Solid-state NMR of proteins sedimented by ultracentrifugation. Proc. Natl. Acad. Sci. USA 108, 10396–10399 (2011).
Ahmad, M.F., Raman, B., Ramakrishna, T. & Rao, C.M. Effect of phosphorylation on αB-crystallin: differences in stability, subunit exchange and chaperone activity of homo and mixed oligomers of αB-crystallin and its phosphorylation-mimicking mutant. J. Mol. Biol. 375, 1040–1051 (2008).
Peschek, J. et al. Regulated structural transitions unleash the chaperone activity of αB-crystallin. Proc. Natl. Acad. Sci. USA 110, E3780–E3789 (2013).
Narayan, P. et al. Amyloid-β oligomers are sequestered by both intracellular and extracellular chaperones. Biochemistry 51, 9270–9276 (2012).
Lee, J., Culyba, E.K., Powers, E.T. & Kelly, J.W. Amyloid-β forms fibrils by nucleated conformational conversion of oligomers. Nat. Chem. Biol. 7, 602–609 (2011).
Meehan, S. et al. Amyloid fibril formation by lens crystallin proteins and its implications for cataract formation. J. Biol. Chem. 279, 3413–3419 (2004).
Laganowsky, A. et al. Atomic view of a toxic amyloid small oligomer. Science 335, 1228–1231 (2012).
Hochberg, G.K.A. et al. The structured core domain of αB-crystallin can prevent amyloid fibrillation and associated toxicity. Proc. Natl. Acad. Sci. USA 111, E1562–E1570 (2014).
Cohen, S.I.A. et al. A molecular chaperone breaks the catalytic cycle that generates toxic Aβ oligomers. Nat. Struct. Mol. Biol. 22, 207–213 (2015).
Klein-Seetharaman, J. et al. Long-range interactions within a nonnative protein. Science 295, 1719–1722 (2002).
Raman, B., Ramakrishna, T. & Rao, C.M. Effect of the chaperone-like alpha-crystallin on the refolding of lysozyme and ribonuclease A. FEBS Lett. 416, 369–372 (1997).
Shi, J. et al. Cryoelectron microscopy analysis of small heat shock protein 16.5 (Hsp16.5) complexes with T4 lysozyme reveals the structural basis of multimode binding. J. Biol. Chem. 288, 4819–4830 (2013).
Jaya, N., Garcia, V. & Vierling, E. Substrate binding site flexibility of the small heat shock protein molecular chaperones. Proc. Natl. Acad. Sci. USA 106, 15604–15609 (2009).
Aquilina, J.A. et al. Subunit exchange of polydisperse proteins: mass spectrometry reveals consequences of alphaA-crystallin truncation. J. Biol. Chem. 280, 14485–14491 (2005).
Aquilina, J.A. et al. Phosphorylation of alphaB-crystallin alters chaperone function through loss of dimeric substructure. J. Biol. Chem. 279, 28675–28680 (2004).
Dasari, M. et al. Bacterial inclusion bodies of Alzheimer's disease β-amyloid peptides can be employed to study native-like aggregation intermediate states. Chembiochem 12, 407–423 (2011).
Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys. J. 78, 1606–1619 (2000).
Bennett, A.E., Rienstra, C.M., Auger, M., Lakshmi, K.V. & Griffin, R.G. Heteronuclear decoupling in rotating solids. J. Chem. Phys. 103, 6951–6958 (1995).
Paulson, E.K. et al. Sensitive high resolution inverse detection NMR spectroscopy of proteins in the solid state. J. Am. Chem. Soc. 125, 15831–15836 (2003).
Zhou, D.H. & Rienstra, C.M. High-performance solvent suppression for proton-detected solid-state NMR. J. Magn. Reson. 192, 167–172 (2008).
Kay, L.E., Torchia, D.A. & Bax, A. Backbone dynamics of proteins as studied by 15N inverse detected heteronuclear NMR spectroscopy: application to staphylococcal nuclease. Biochemistry 28, 8972–8979 (1989).
Choy, W.-Y. et al. Distribution of molecular size within an unfolded state ensemble using small-angle X-ray scattering and pulse field gradient NMR techniques. J. Mol. Biol. 316, 101–112 (2002).
Marsh, J.A., Singh, V.K., Jia, Z. & Forman-Kay, J.D. Sensitivity of secondary structure propensities to sequence differences between alpha- and gamma-synuclein: implications for fibrillation. Protein Sci. 15, 2795–2804 (2006).
Comeau, S.R., Gatchell, D.W., Vajda, S. & Camacho, C.J. ClusPro: an automated docking and discrimination method for the prediction of protein complexes. Bioinformatics 20, 45–50 (2004).
Acknowledgements
We are grateful to H. Oschkinat, S. Markovic and J. Muenckemer for stimulating discussions on the project. We also thank M. Ringling, H. Stephanowitz and A. Nieuwkoop for technical support with the EM, MS and fast MAS NMR experiments, respectively. This work was performed in the framework of the SFB-1035 (project B07 to B.R. and project A06 to J.B. and S.W.; German Research Foundation (DFG)). We acknowledge support from the Helmholtz-Gemeinschaft and the DFG (grant Re1435 to B.R.). We are grateful to the Center for Integrated Protein Science Munich for financial support. J.P. acknowledges support from the Studienstiftung des deutschen Volkes.
Author information
Authors and Affiliations
Contributions
A.M., J.P., M.S. and K.C.B. cloned, recombinantly expressed, purified and characterized human αB and its variants. A.M. performed the NMR experiments and analyzed the data. J.P. performed the lysozyme aggregation assays. K.C.B. performed the seeded amyloid aggregation assays and characterized the variant αB S135Q. M.S. and S.A. contributed the diffusion-ordered spectroscopy and proton-driven spin diffusion NMR data. B.B. generated the docking model of αB and fibrillar Aβ1–40. E.P. prepared recombinant Aβ1–40 and supported the resonance assignment of Aβ1–40 in solution. C.P. performed the EM experiments of αB and lysozyme. A.M., J.P., J.B., S.W. and B.R. designed the experiments. A.M. and B.R. wrote the manuscript, with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Identification of intersubunit contacts in αB oligomers.
(a) Overlay of 1H-15N correlation spectra of full-length αB in the sedimented state (red) and the dimeric core domain αB10m in solution (blue). Resonance assignments are given for the full-length protein. Arrows indicate the strongest chemical shift changes. (b) Chemical shift differences (Δδ) between αB oligomers (major species) and αB10m dimers as extracted from the spectra shown in (a) versus the ACD sequence. A similar comparison has been shown previously by Jehle, S. et al., J. Mol. Biol. 385, 1481–1497, 2009. Residues showing the largest effects are highlighted in red. Note that αB10m harbors the mutation N146D. Asterisks denote either proline residues or residues which are not assigned for full-length αB. Secondary structure elements from NMR data (Mainz, A. et al., J. Biol. Chem. 287, 1128–1138, 2012) are indicated on top. Highlighted are intermolecular interfaces of the ACD (up to 10 Å) as observed in the oligomer models by NMR (Jehle, S. et al., J. Mol. Biol. 385, 1481–1497, 2009; Jehle, S. et al., Nat. Struct. Mol. Biol. 17, 1037–1042, 2010) (green boxes) and by cryo-EM (Braun, N. et al., Proc. Natl. Acad. Sci. U. S. A. 108, 20491–20496, 2011) (blue boxes), respectively. Blank boxes indicate intramolecular contacts in the oligomer models. For comparison, panel f of Fig. 1 of the main text is depicted on top, highlighting assigned residues (light blue) and residues showing peak doubling (red).
Supplementary Figure 2 Spatial vicinity between protons in αB, as observed by RFDR MAS NMR spectroscopy.
(a) Section of the 1H-15N correlation MAS NMR spectrum of perdeuterated αB (black) showing the two cross-peaks for I133 (A and B). The corresponding 2D strips of the 3D hCXhNH spectra (red) demonstrate the degeneracy in the 13C dimension for both states and thus the benefits of introducing the 1H dimension to resolve the two structural states. 2D strips of the 3D 1H-1H RFDR spectrum (green) show the spatial connectivities between the backbone amides of I133-A and -B and the neighboring loop residues (including side-chain hydroxyl groups). The asterisks denote diagonal peaks. Both signals of I133 are devoid of exchange peaks with bulk water (4.7 ppm), which is in contrast e.g. to the more solvent-accessible loop residues S85–L89 (see panel f). (b) The asymmetric dimer interface II in the structural model of Braun, N. et al., Proc. Natl. Acad. Sci. U. S. A. 108, 20491–20496, 2011, and the different locations of residue I133 (magenta). The two protomers are colored in white (extended conformer) and green (bent conformer), respectively. The two-fold point-symmetry of the ACD dimer is disturbed by the differential arrangement of the corresponding NTDext/CTDext and NTDbent/CTDbent, respectively. (c) Close-up view of the loop residues preceeding I133 in the dimer structure of αB (Jehle, S. et al., Nat. Struct. Mol. Biol. 17, 1037–1042, 2010; Braun, N. et al., Proc. Natl. Acad. Sci. U. S. A. 108, 20491–20496, 2011). The spatial vicinity between the amide protons of I133, T132, L131 and D129 is confirmed by the observed RFDR contacts in (a). (d) Different register of the dimer interface I as observed in different X-ray structures of the excised αB dimer (Bagnéris, C. et al., J. Mol. Biol. 392, 1242–1252, 2009; Laganowsky, A. et al., Protein Sci. 19, 1031–1043, 2010). The interfaces are termed API and APII register and have the point-symmetric axis centered at residue F118 and E117, respectively. (e) 2D strips extracted from the 3D 1H-1H RFDR MAS NMR spectrum of αB. The strip plot shows inter-strand correlations between residues S115 and H119 (β6+7) supporting an APII register in full-length αB as indicated in the red box in (d). (f) Spatial vicinity between loop residues S85–L89. 2D strips extracted from 3D 1H-1H RFDR MAS NMR spectra recorded at 40 kHz MAS. Spatial contacts are indicated by dashed lines to guide the eye. Asterisks denote diagonal peaks. (g) Structure of the loop connecting strands β3 and β4 (residues S85–L89) in αB according to Braun, N. et al., Proc. Natl. Acad. Sci. U. S. A. 108, 20491–20496, 2011. (h) Imidazole region of 2D 1H-15N correlation spectra recorded on full-length αB (CP-MAS, red) and αB10m (solution-state HMQC, black). The two resonances of H104 (A and B) reveal a comparable signal pattern in strips of the 3D 1H-1H RFDR spectrum in (i). The spatial vicinity to the backbone amide of R116 represents a strand-strand contact involving β5 and β6+7. (j) Residues close to the side-chain of H104 in the structural model of αB according to Braun, N. et al., Proc. Natl. Acad. Sci. U. S. A. 108, 20491–20496, 2011.
Supplementary Figure 3 Differential behavior of N- and C-terminal regions in αB.
(a) Accessibility of N- and C-terminal proteolytic cleavage-sites in αB. SDS-PAGE of His6-Ek–αB (25 kDa, where Ek denotes the recognition site of the protease enterokinase) before (lane 1), after 15 h (lane 2) and after 40 h (lane 3) incubation with enterokinase at 4 °C. Molecular weights of relevant marker bands (M) are indicated. The asterisks label the non-specific by-products, which were analyzed by (b) MALDI-TOF MS of the protein solution after enterokinase degradation and (c) MALDI-TOF/TOF MS2 of the corresponding gel bands of αB (blue) and αB* (red) after tryptic digest. The MS results clearly identify the by-product as a truncation variant missing the C-terminal residues T158–K175 (1,943 Da). (d) Enlarged view of the αB 24-mer5 showing three bent conformers (blue) and three extended conformers (red) participating in the intermolecular packing of the six NTDs at the so-called “closed” three-fold point-symmetric axis (C3closed). The “open” three-fold point-symmetric axis (C3open) centered in the hexameric ring is also indicated. The locations of the N- and C-termini of bent and extended conformers are highlighted to visualize the different accessibilities of the termini: the bent conformer exposes both termini towards the surface of the oligomer, whereas the extended conformer locates both termini close to each other in the interior of the assembly. This asymmetric arrangement is supported by the occurrence of specific and non-specific processing at the dissimilar N- and C-termini, respectively. (e) Diffusion attenuation profiles for the major (black) and minor conformers (red) of the αB CTD are plotted for residues R157, A172 and K175. In all cases, both conformers decay equally. The average translational diffusion coefficient amounts to 2.65 (± 0.16) × 10−11 m2/s. The profiles were recorded at 295 K and an external magnetic field of 21.1 T. (f) Simulation of attenuation profiles (dashed lines) for various αB n-mers as indicated in the legend. Data points of the experimental profiles are shown for both conformers of K175.
Supplementary Figure 4 Interaction studies with αB and nonreduced lysozyme.
(a) Sedimentation velocity experiments using αB–WT (red), lysozyme (blue) and an equimolar mixture of αB–WT and lysozyme (black) under non-reducing conditions. The inset shows the broad distribution of αB oligomers. No aggregation of non-reduced lysozyme and no interaction with αB are observed at the low protein concentration used in this experiment (20 µM). (b) Top: Threonine (left) and isoleucine spectral region (right) of 13C-13C correlation MAS NMR spectra of αB (5.5 mM) in the absence (red) and presence of stoichiometric amounts of non-reduced (native) lysozyme (black). Only small chemical-shift changes are observed and might be attributed to a small fraction of lysozyme aggregates due the high protein concentrations used in these experiments (5.5 mM). Bottom: the equivalent experiment with reduced (unfolded) lysozyme is shown for comparison (see Fig. 3c of main text).
Supplementary Figure 5 2D MAS NMR spectra of αB and its lysozyme-bound state.
(a) Aliphatic region of solid-state MAS 13C-13C correlation spectra of αB in the absence and presence of reduced lysozyme. Concentrated solutions of αB (red) and αB:lysozyme in a molar ratio of 7:1 (blue) were filled directly into the MAS rotor. Protein immobilization was accomplished by MAS-induced protein sedimentation (Mainz, A., Jehle, S., van Rossum, B. J., Oschkinat, H. & Reif, B., J. Am. Chem. Soc. 131, 15968–15969, 2009; Bertini, I. et al., Proc. Natl. Acad. Sci. 108, 10396–10399, 2011). At an equimolar ratio of αB and reduced lysozyme (black), the resulting co-precipitate was filled as such into the MAS rotor. (b) 1H-15N correlation spectra of αB in the absence (red) and presence of stoichiometric amounts of reduced lysozyme (blue). To record the αB reference spectrum, a concentrated solution of αB was filled into the MAS rotor. By contrast, the equimolar mixture of αB and reduced lysozyme was yielding a co-precipitate that was filled as such into the MAS rotor. Resonance assignments are shown for αB in the absence of client.
Supplementary Figure 6 Dimeric αB10m does not act as a holdase during lysozyme aggregation.
(a) 1H-15N SOFAST-HMQC spectra of 200 μM 15N-labeled αB10m in the presence of 400 μM unlabeled lysozyme (blue) and after 3 h incubation of this mixture with a 20-fold molar excess of the reducing agent TCEP (black contour) at 22 °C. Both spectra are drawn using the same contour level. Representative 1D traces along the 1H dimension of both spectra at 15N chemical shifts corresponding to the cross-peaks of I133 and L94, respectively, are shown in (b). The same color code as in (a) is used. Upon aggregation of lysozyme there is no change in chemical shift, signal intensity or line width for the resonances of αB10m, which shows that the excised ACD dimer does not interact neither with misfolded lysozyme monomers nor with its aggregates. (c) Down-field region of 1D 1H spectra of the same samples showing exclusively lysozyme resonances in the presence of αB10m at several time points after addition of TCEP.
Supplementary Figure 7 Prediction of hydrophobicity and structural disorder of αB WT.
The hydrophobicity score (red graph) was calculated according to Eisenberg, D., Schwarz, E., Komaromy, M. & Wall, R., J. Mol. Biol. 179, 125–142, 1984. The propensity for structural disorder (blue graph) was predicted using the webserver PSIPRED (Buchan, D. W. A., Minneci, F., Nugent, T. C. O., Bryson, K. & Jones, D. T., Nucleic Acids Res. 41, W349–W357, 2013). Domain topology and secondary structure elements as observed by solid-state NMR (Jehle, S. et al., J. Mol. Biol. 385, 1481–1497, 2009; Jehle, S. et al., Nat. Struct. Mol. Biol. 17, 1037–1042, 2010; Jehle, S. et al., Proc. Natl. Acad. Sci. U. S. A. 108, 6409–6414, 2011) are shown on top. Hydrophobic regions are highlighted in red, whereas potentially disordered and ordered regions (as predicted by GlobPlot according to Linding, R., Russell, R. B., Neduva, V. & Gibson, T. J., Nucleic Acids Res. 31, 3701–3708, 2003) are indicated with blue and green boxes, respectively. The sequence of the NTD is given on top with the nine proline residues highlighted in blue. Two proline-rich clusters can be identified, both harboring serine residues that are known to be phosphorylated in vivo (orange) with concomitant impact on the quaternary structure and chaperone activity of αB (Aquilina, J. A. et al., J. Biol. Chem. 279, 28675–28680, 2004); Peschek, J. et al., Proc. Natl. Acad. Sci. U. S. A. 110, E3780–3789, 2013).
Supplementary Figure 8 Aggregation of Aβ1–40 and its propensity for β-strand conformation.
(a) Aβ1–40 aggregation kinetics in the absence (red) and presence of sub-stoichiometric amounts of αB (black) monitored by dynamic light scattering. Mean count rate (MCR) in kilo counts per second (kcps) is a measure of sample turbidity. Size distributions are shown in (b) for the initial (left) and final stage (right, after 4 d) of the experiment. The time-dependent changes for the oligomer A are shown in Figure 4b of the main manuscript. (c) Secondary structure propensity (SSP) of the monomeric Aβ1–40 peptide in aqueous solution at pH 7.4. The SSP score was determined as described previously by Marsh, J. A., Singh, V. K., Jia, Z. & Forman-Kay, J. D., Protein Sci. Publ. Protein Soc. 15, 2795–2804, 2006, and reflects the presence of secondary structure (α-helical for SSP > 0.1; β-strand for SSP < –0.1) in a sub-population of the molecule under investigation. The regions of monomeric Aβ1–40, which show β-strand propensity, are highlighted in blue. The amino acid sequence of Aβ1–40 is shown on top. The asterisks denote non-assigned residues. The hydrophobic core region L17–A21 involved in αB interaction (Narayanan, S., Kamps, B., Boelens, W. C. & Reif, B., FEBS Lett. 580, 5941–5946, 2006) is highlighted in red. β-strand conformation observed in the structural model of Aβ1–40 fibrils (Paravastu, A. K., Leapman, R. D., Yau, W.-M. & Tycko, R., Proc. Natl. Acad. Sci. U. S. A. 105, 18349–18354, 2008) and in the fibril structure of Aβ1-42–E22Δ (Schütz, A. K. et al., Angew. Chem. Int. Ed. 54, 331–335, 2015) is indicated on top in blue and magenta, respectively.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Tables 1–3 and Supplementary Note (PDF 3875 kb)
Supplementary Data Set 1
Uncropped image of the SDS-PAGE gel shown in Figure 3a (PDF 99 kb)
Supplementary Data Set 2
Coordinates of the docking model (PDF 1292 kb)
Rights and permissions
About this article
Cite this article
Mainz, A., Peschek, J., Stavropoulou, M. et al. The chaperone αB-crystallin uses different interfaces to capture an amorphous and an amyloid client. Nat Struct Mol Biol 22, 898–905 (2015). https://doi.org/10.1038/nsmb.3108
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3108
This article is cited by
-
Short hydrophobic loop motifs in BRICHOS domains determine chaperone activity against amorphous protein aggregation but not against amyloid formation
Communications Biology (2023)
-
Is the lipochaperone activity of sHSP a key to the stress response encoded in its primary sequence?
Cell Stress and Chaperones (2023)
-
Structural basis for the inhibition of IAPP fibril formation by the co-chaperonin prefoldin
Nature Communications (2022)
-
Solid-state NMR spectroscopy
Nature Reviews Methods Primers (2021)
-
O-GlcNAc modification of small heat shock proteins enhances their anti-amyloid chaperone activity
Nature Chemistry (2021)