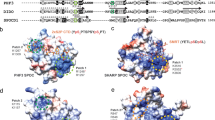
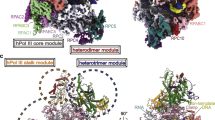
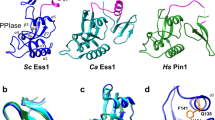
Abstract
The RNA polymerase II (RNAPII) C-terminal domain (CTD) heptapeptide repeats (1-YSPTSPS-7) undergo dynamic phosphorylation and dephosphorylation during the transcription cycle to recruit factors that regulate transcription, RNA processing and chromatin modification. We show here that RPRD1A and RPRD1B form homodimers and heterodimers through their coiled-coil domains and interact preferentially via CTD-interaction domains (CIDs) with RNAPII CTD repeats phosphorylated at S2 and S7. Crystal structures of the RPRD1A, RPRD1B and RPRD2 CIDs, alone and in complex with RNAPII CTD phosphoisoforms, elucidate the molecular basis of CTD recognition. In an example of cross-talk between different CTD modifications, our data also indicate that RPRD1A and RPRD1B associate directly with RPAP2 phosphatase and, by interacting with CTD repeats where phospho-S2 and/or phospho-S7 bracket a phospho-S5 residue, serve as CTD scaffolds to coordinate the dephosphorylation of phospho-S5 by RPAP2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Allison, L.A., Moyle, M., Shales, M. & Ingles, C.J. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell 42, 599–610 (1985).
Corden, J.L. Tails of RNA polymerase II. Trends Biochem. Sci. 15, 383–387 (1990).
Egloff, S., Dienstbier, M. & Murphy, S. Updating the RNA polymerase CTD code: adding gene-specific layers. Trends Genet. 28, 333–341 (2012).
Singh, N. et al. The Ess1 prolyl isomerase is required for transcription termination of small noncoding RNAs via the Nrd1 pathway. Mol. Cell 36, 255–266 (2009).
Sims, R.J. III et al. The C-terminal domain of RNA polymerase II is modified by site-specific methylation. Science 332, 99–103 (2011).
Buratowski, S. The CTD code. Nat. Struct. Biol. 10, 679–680 (2003).
Corden, J.L. Transcription: seven ups the code. Science 318, 1735–1736 (2007).
Hsin, J.P., Sheth, A. & Manley, J.L. RNAP II CTD phosphorylated on threonine-4 is required for histone mRNA 3′ end processing. Science 334, 683–686 (2011).
Krogan, N.J. et al. The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: linking transcriptional elongation to histone methylation. Mol. Cell 11, 721–729 (2003).
Mayer, A. et al. CTD tyrosine phosphorylation impairs termination factor recruitment to RNA polymerase II. Science 336, 1723–1725 (2012).
Kim, M., Suh, H., Cho, E.J. & Buratowski, S. Phosphorylation of the yeast Rpb1 C-terminal domain at serines 2, 5, and 7. J. Biol. Chem. 284, 26421–26426 (2009).
Glover-Cutter, K. et al. TFIIH-associated Cdk7 kinase functions in phosphorylation of C-terminal domain Ser7 residues, promoter-proximal pausing, and termination by RNA polymerase II. Mol. Cell. Biol. 29, 5455–5464 (2009).
Akhtar, M.S. et al. TFIIH kinase places bivalent marks on the carboxy-terminal domain of RNA polymerase II. Mol. Cell 34, 387–393 (2009).
Chapman, R.D. et al. Transcribing RNA polymerase II is phosphorylated at CTD residue serine-7. Science 318, 1780–1782 (2007).
Serizawa, H. et al. Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature 374, 280–282 (1995).
Ghosh, A., Shuman, S. & Lima, C.D. Structural insights to how mammalian capping enzyme reads the CTD code. Mol. Cell 43, 299–310 (2011).
McCracken, S. et al. 5′-capping enzymes are targeted to pre-mRNA by binding to the phosphorylated carboxy-terminal domain of RNA polymerase II. Genes Dev. 11, 3306–3318 (1997).
Cho, E.J., Takagi, T., Moore, C.R. & Buratowski, S. mRNA capping enzyme is recruited to the transcription complex by phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 11, 3319–3326 (1997).
Fabrega, C., Shen, V., Shuman, S. & Lima, C.D. Structure of an mRNA capping enzyme bound to the phosphorylated carboxy-terminal domain of RNA polymerase II. Mol. Cell 11, 1549–1561 (2003).
Ng, H.H., Robert, F., Young, R.A. & Struhl, K. Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol. Cell 11, 709–719 (2003).
Krogan, N.J. et al. COMPASS, a histone H3 (lysine 4) methyltransferase required for telomeric silencing of gene expression. J. Biol. Chem. 277, 10753–10755 (2002).
Briggs, S.D. et al. Histone H3 lysine 4 methylation is mediated by Set1 and required for cell growth and rDNA silencing in Saccharomyces cerevisiae. Genes Dev. 15, 3286–3295 (2001).
Vasiljeva, L., Kim, M., Mutschler, H., Buratowski, S. & Meinhart, A. The Nrd1–Nab3–Sen1 termination complex interacts with the Ser5-phosphorylated RNA polymerase II C-terminal domain. Nat. Struct. Mol. Biol. 15, 795–804 (2008).
Mutskov, V.J., Farrell, C.M., Wade, P.A., Wolffe, A.P. & Felsenfeld, G. The barrier function of an insulator couples high histone acetylation levels with specific protection of promoter DNA from methylation. Genes Dev. 16, 1540–1554 (2002).
Zeitlinger, J. et al. RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nat. Genet. 39, 1512–1516 (2007).
Guenther, M.G., Levine, S.S., Boyer, L.A., Jaenisch, R. & Young, R.A. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 130, 77–88 (2007).
Core, L.J., Waterfall, J.J. & Lis, J.T. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 322, 1845–1848 (2008).
Cho, E.J., Kobor, M.S., Kim, M., Greenblatt, J. & Buratowski, S. Opposing effects of Ctk1 kinase and Fcp1 phosphatase at Ser 2 of the RNA polymerase II C-terminal domain. Genes Dev. 15, 3319–3329 (2001).
Zhou, Q., Li, T. & Price, D.H. RNA polymerase II elongation control. Annu. Rev. Biochem. 81, 119–143 (2012).
Bartkowiak, B. et al. CDK12 is a transcription elongation-associated CTD kinase, the metazoan ortholog of yeast Ctk1. Genes Dev. 24, 2303–2316 (2010).
Devaiah, B.N. et al. BRD4 is an atypical kinase that phosphorylates serine2 of the RNA polymerase II carboxy-terminal domain. Proc. Natl. Acad. Sci. USA 109, 6927–6932 (2012).
Mosley, A.L. et al. Rtr1 is a CTD phosphatase that regulates RNA polymerase II during the transition from serine 5 to serine 2 phosphorylation. Mol. Cell 34, 168–178 (2009).
Egloff, S., Zaborowska, J., Laitem, C., Kiss, T. & Murphy, S. Ser7 phosphorylation of the CTD recruits the RPAP2 Ser5 phosphatase to snRNA genes. Mol. Cell 45, 111–122 (2012).
Bataille, A.R. et al. A universal RNA polymerase II CTD cycle is orchestrated by complex interplays between kinase, phosphatase, and isomerase enzymes along genes. Mol. Cell 45, 158–170 (2012).
Hausmann, S., Koiwa, H., Krishnamurthy, S., Hampsey, M. & Shuman, S. Different strategies for carboxyl-terminal domain (CTD) recognition by serine 5-specific CTD phosphatases. J. Biol. Chem. 280, 37681–37688 (2005).
Krishnamurthy, S., He, X., Reyes-Reyes, M., Moore, C. & Hampsey, M. Ssu72 Is an RNA polymerase II CTD phosphatase. Mol. Cell 14, 387–394 (2004).
Archambault, J. et al. FCP1, the RAP74-interacting subunit of a human protein phosphatase that dephosphorylates the carboxyl-terminal domain of RNA polymerase IIO. J. Biol. Chem. 273, 27593–27601 (1998).
Kobor, M.S. et al. An unusual eukaryotic protein phosphatase required for transcription by RNA polymerase II and CTD dephosphorylation in S. cerevisiae. Mol. Cell 4, 55–62 (1999).
Xiang, K., Manley, J.L. & Tong, L. The yeast regulator of transcription protein Rtr1 lacks an active site and phosphatase activity. Nat. Commun. 3, 946 (2012).
Lu, D. et al. CREPT accelerates tumorigenesis by regulating the transcription of cell-cycle-related genes. Cancer Cell 21, 92–104 (2012).
Ni, Z. et al. Control of the RNA polymerase II phosphorylation state in promoter regions by CTD interaction domain-containing proteins RPRD1A and RPRD1B. Transcription 2, 237–242 (2011).
Meinhart, A. & Cramer, P. Recognition of RNA polymerase II carboxy-terminal domain by 3′-RNA-processing factors. Nature 430, 223–226 (2004).
Lunde, B.M. et al. Cooperative interaction of transcription termination factors with the RNA polymerase II C-terminal domain. Nat. Struct. Mol. Biol. 17, 1195–1201 (2010).
Liu, Y. & Eisenberg, D. 3D domain swapping: as domains continue to swap. Protein Sci. 11, 1285–1299 (2002).
Nooren, I.M., Kaptein, R., Sauer, R.T. & Boelens, R. The tetramerization domain of the Mnt repressor consists of two right-handed coiled coils. Nat. Struct. Biol. 6, 755–759 (1999).
Lindell, T.J., Weinberg, F., Morris, P.W., Roeder, R.G. & Rutter, W.J. Specific inhibition of nuclear RNA polymerase II by α-amanitin. Science 170, 447–449 (1970).
Stiller, J.W. & Cook, M.S. Functional unit of the RNA polymerase II C-terminal domain lies within heptapeptide pairs. Eukaryot. Cell 3, 735–740 (2004).
Noble, C.G., Walker, P.A., Calder, L.J. & Taylor, I.A. Rna14-Rna15 assembly mediates the RNA-binding capability of Saccharomyces cerevisiae cleavage factor IA. Nucleic Acids Res. 32, 3364–3375 (2004).
Czudnochowski, N., Bosken, C.A. & Geyer, M. Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition. Nat. Commun. 3, 842 (2012).
Wang, B.C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 115, 90–112 (1985).
Hendrickson, W.A., Horton, J.R. & LeMaster, D.M. Selenomethionyl proteins produced for analysis by multiwavelength anomalous diffraction (MAD): a vehicle for direct determination of three-dimensional structure. EMBO J. 9, 1665–1672 (1990).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Lebedev, A.A. et al. JLigand: a graphical tool for the CCP4 template-restraint library. Acta Crystallogr. D Biol. Crystallogr. 68, 431–440 (2012).
Moffat, J. et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124, 1283–1298 (2006).
Mak, A.B. et al. A lentiviral functional proteomics approach identifies chromatin remodeling complexes important for the induction of pluripotency. Mol. Cell. Proteomics 9, 811–823 (2010).
Ni, Z., Olsen, J.B., Emili, A. & Greenblatt, J.F. Identification of mammalian protein complexes by lentiviral-based affinity purification and mass spectrometry. Methods Mol. Biol. 781, 31–45 (2011).
Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 78, 1606–1619 (2000).
Acknowledgements
We thank D.Y. Zhao, X. Wu and K. Liu for technical assistance, J. Moffat (University of Toronto) for providing the shRNAs and D. Eick (Helmholtz-Zentrum, Munich) for providing antibodies. This work was supported by grants from the Canadian Institutes of Health Research (CIHR MOP-258357) (to J.F.G. and A.E.), the Ontario Research Fund (to J.F.G. and A.E.), the US National Institutes of Health (GM099714) (to A.L.M.), the Wellcome Trust (092483/Z/10/Z), Edward Penley Abraham (to S.M.) and the Canada Foundation for Innovation (CFI) (to J.F.G. and A.E.), and by a CIHR Postdoctoral Fellowship (to Z.N.), Oxford Stem Cell Institute Studentship (to O.V.K.) and Ontario Graduate Scholarship (to J.B.O.). The Structural Genomics Consortium is a registered charity (number 1097737) that receives funds from AbbVie, Boehringer Ingelheim, CFI, CIHR, Genome Canada through the Ontario Genomics Institute (OGI-055), GlaxoSmithKline, Janssen, Lilly Canada, the Novartis Research Foundation, the Ontario Ministry of Economic Development and Innovation, Pfizer, Takeda and the Wellcome Trust (092809/Z/10/Z) (to C.H.A. and J.M.). Results in this report are partially derived from work performed at Argonne National Laboratory, Structural Biology Center at the Advanced Photon Source. Argonne is operated by University of Chicago, Argonne, for the US Department of Energy, Office of Biological and Environmental Research, under contract DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
Z.N. and J.F.G. conceived the project and designed the experiments. Z.N. and C.X. performed protein expression, purification and crystallization experiments and analyzed the structure data. W.T. and M.E.B. conducted crystallographic data collection, structure determination and refinement. Z.N., X.G., G.O.H., O.V.K., E.M., G.Z., H.G., W.-H.W.K., J.L., P.Y., J.B.O., C.W., P.L., G.A.S., H. He and H. Huang conducted experiments. S.S.S., A.E., S.M., A.L.M., C.H.A. and J.M. guided the experiments. All authors commented on the manuscript. Z.N. analyzed the data. Z.N. and J.F.G. wrote the manuscript. J.M. and J.F.G. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 2 Dimerization of RPRDs. Related to Figure 4.
(a) Size exclusion chromatography analysis of the indicated recombinant proteins purified from E. coli. (b) Dynamic light scattering measurement of the molecular weights of the indicated recombinant proteins purified from E. coli. The ratios of measured vs monomer molecular weight are shown at the top of each bar. Error bars: s.d. from three technical replicates. (c) SDS-polyacrylamide gel analysis showing the indicated recombinant proteins cross-linked with the indicated concentration of suberic acid-bis-(3-sulfo-N-hydroxysuccinimide ester) (BS3). Putative multimers are labeled.
Supplementary Figure 3 RPRD1A interacts with RPRD1B via coiled-coil domain in vitro. Related to Figure 4.
Glutathione-S-transferase (GST) pull-down showing the interactions between GST-tagged RPRD1A and His-tagged RPRD1B, its CID or its coiled-coil domain expressed in E. coli.
Supplementary Figure 4 RPAP2 phosphatase activity in vitro. Related to Figure 6.
(a) In vitro phosphatase assays using 6,8-difluoro-4-methylumbelliferyl phosphate (DiFMUP) substrate showing the effects of RPRD1A and RPRD1B on RPAP2 (1-334) activity. The reaction includes 1 μM RPAP2 (1-334), 10 μM DiFMUP and indicated concentration of RPRD1A or RPRD1B. The fluorescence level induced by RPAP2 (1-334) was set as 100%. Error bars: s.d. from four technical replicates. (b) In vitro DiFMUP phosphatase assays showing the effects on RPAP2 phosphatase activity by the phosphatase inhibitor vanadate. RPAP2 (1-334): 2 μM. DiFMUP: 10 μM. Vanadate: 1 mM. The fluorescence level induced by RPAP2 (1-334) was set as 100%. *: p<0.01 as compared with the no inhibitor controls (two-tailed Student’s t-test). Error bars: s.d. from five technical replicates. (c) In vitro DiFMUP phosphatase assays showing the effects on RPAP2 phosphatase activity by indicated metals. RPAP2 (1-334): 2 μM. DiFMUP: 10 μM. Indicated metals: 10 mM. The fluorescence level induced by RPAP2 (1-334) in the absence of added metals was set as 100%. *: p<0.01 as compared with the no metal controls (one-way ANOVA Tukey's Multiple Comparison test). Error bars: s.d. from six technical replicates. (d) In vitro phosphatase ELISAs showing the effects of vanadate and the indicated metals on RPRD1A-stimulated RPAP2 phosphatase activity on the indicated biotinylated CTD peptide. The reactions contain three µM RPAP2, 10 μM RPRD1A and biotinylated S2-5-2P CTD peptide, with or without 1 mM vanadate or 10 mM of the indicated metals, incubated at 37 °C for 3 hr, followed by measurement of the phosphorylation levels using the S5P monoclonal antibody (3E8). No protein controls were set as 100%. *: p<0.01 as compared with the no inhibitor controls (two-tailed Student’s t-test). Error bars; s.d. from four technical replicates. (e) In vitro phosphatase ELISAs showing the effects on the antibody recognition at S5P by adjacent S7P. The reactions contain the indicated biotinylated CTD peptides incubated with 3 µM RPAP2 and 10 µM RPRD1A at 37 °C for 3 hours. Phosphorylation levels were measured using the indicated antibodies. *: p<0.01 (two-tailed Student’s t-test). Error bars: s.d. from three technical replicates. (f) In vitro phosphatase ELISAs assays showing the effects of RPRD1A or RPRD1B stimulating RPAP2 phosphatase activity on S5P located between two S7P-containing CTD repeats. The reactions contain the indicated biotinylated CTD peptides, indicated proteins incubated at 37 °C for 3 hours, followed by measurement of the phosphorylation levels using the indicated antibodies. No protein controls were set as 100%. *: p<0.01 (two-tailed Student’s t-test) as compared to no protein control. Error bars: s.d. from four technical replicates.
Supplementary Figure 5 Regulation of the phosphorylation level of CTD S5 in vivo. Related to Figure 6.
(a) Chromatin immunoprecipitation (ChIP) experiments using the indicated antibodies showing the effects on S5P levels at various promoters by shRNA-mediated knock-down of RPAP2 in HEK293 cells. *: p < 0.05 compared with shGFP (two-tailed Student’s t-test). Error bars: s.d. from three biological replicates. (b) Western blotting using the indicated antibodies showing the effects on the global S5P and S2P levels by shRNA-mediated knock-down of RPAP2 in HEK293 cells. WT: no lentiviral shRNA infected cells. (c) ChIP experiments using the indicated antibodies showing the effects on S5P levels at various promoters or near promoter regions by siRNA-mediated knocking down RPRD1A in HeLa cell extracts. ACTB (β-ACTIN ): +300 bp region. The S5P levels were normalized to those of RNAPII (detected by N20 antibody as in Fig. 5e). *: p < 0.05 compared with no siRNA transfection control (two-tailed Student’s t-test). Error bars: s.d. from three biological replicates. The bar for β -ACTIN indicates the range of two biological replicates.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1–4 (PDF 1227 kb)
Supplementary Data Set
Original gel images for western blots (PDF 1030 kb)
Rights and permissions
About this article
Cite this article
Ni, Z., Xu, C., Guo, X. et al. RPRD1A and RPRD1B are human RNA polymerase II C-terminal domain scaffolds for Ser5 dephosphorylation. Nat Struct Mol Biol 21, 686–695 (2014). https://doi.org/10.1038/nsmb.2853
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2853
This article is cited by
-
Super enhancers targeting ZBTB16 in osteogenesis protect against osteoporosis
Bone Research (2023)
-
Current understanding of CREPT and p15RS, carboxy-terminal domain (CTD)-interacting proteins, in human cancers
Oncogene (2021)
-
CREPT is required for murine stem cell maintenance during intestinal regeneration
Nature Communications (2021)
-
CREPT/RPRD1B promotes tumorigenesis through STAT3-driven gene transcription in a p300-dependent manner
British Journal of Cancer (2021)
-
TASOR is a pseudo-PARP that directs HUSH complex assembly and epigenetic transposon control
Nature Communications (2020)