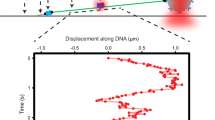
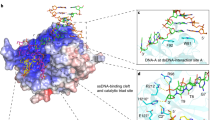
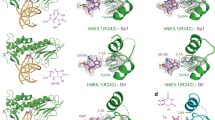
Abstract
ALKBH2 is a direct DNA repair dioxygenase guarding the mammalian genome against N1-methyladenine, N3-methylcytosine and 1,N6-ethenoadenine damage. A prerequisite for repair is to identify these lesions in the genome. Here we present crystal structures of human ALKBH2 bound to different duplex DNAs. Together with computational and biochemical analyses, our results suggest that DNA interrogation by ALKBH2 has two previously unknown features: (i) ALKBH2 probes base-pair stability and detects base pairs with reduced stability, and (ii) ALKBH2 does not have nor need a damage-checking site, which is critical for preventing spurious base cleavage for several glycosylases. The demethylation mechanism of ALKBH2 insures that only cognate lesions are oxidized and reversed to normal bases, and that a flipped, non-substrate base remains intact in the active site. Overall, the combination of duplex interrogation and oxidation chemistry allows ALKBH2 to detect and process diverse lesions efficiently and correctly.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sedgwick, B. Repairing DNA-methylation damage. Nat. Rev. Mol. Cell Biol. 5, 148–157 (2004).
Sedgwick, B., Bates, P.A., Paik, J., Jacobs, S.C. & Lindahl, T. Repair of alkylated DNA: Recent advances. DNA Repair (Amst.) 6, 429–442 (2007).
Yi, C., Yang, C.G. & He, C. A non-heme iron-mediated chemical demethylation in DNA and RNA. Acc. Chem. Res. 42, 519–529 (2009).
Duncan, T. et al. Reversal of DNA alkylation damage by two human dioxygenases. Proc. Natl. Acad. Sci. USA 99, 16660–16665 (2002).
Aas, P.A. et al. Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature 421, 859–863 (2003).
Ringvoll, J. et al. Repair deficient mice reveal mABH2 as the primary oxidative demethylase for repairing 1meA and 3meC lesions in DNA. EMBO J. 25, 2189–2198 (2006).
Ringvoll, J. et al. AlkB homologue 2-mediated repair of ethenoadenine lesions in mammalian DNA. Cancer Res. 68, 4142–4149 (2008).
Cetica, V. et al. Pediatric brain tumors: mutations of two dioxygenases (hABH2 and hABH3) that directly repair alkylation damage. J. Neurooncol. 94, 195–201 (2009).
Wu, S.S. et al. Down-regulation of ALKBH2 increases cisplatin sensitivity in H1299 lung cancer cells. Acta Pharmacol. Sin. 32, 393–398 (2011).
Gilljam, K.M. et al. Identification of a novel, widespread, and functionally important PCNA-binding motif. J. Cell Biol. 186, 645–654 (2009).
Yang, C.G. et al. Crystal structures of DNA/RNA repair enzymes AlkB and ABH2 bound to dsDNA. Nature 452, 961–965 (2008).
Huffman, J.L., Sundheim, O. & Tainer, J.A. DNA base damage recognition and removal: new twists and grooves. Mutat. Res. 577, 55–76 (2005).
Yang, C.G., Garcia, K. & He, C. Damage detection and base flipping in direct DNA alkylation repair. ChemBioChem 10, 417–423 (2009).
Lau, A.Y., Wyatt, M.D., Glassner, B.J., Samson, L.D. & Ellenberger, T. Molecular basis for discriminating between normal and damaged bases by the human alkyladenine glycosylase, AAG. Proc. Natl. Acad. Sci. USA 97, 13573–13578 (2000).
Wolfe, A.E. & O'Brien, P.J. Kinetic mechanism for the flipping and excision of 1,N6-ethenoadenine by human alkyladenine DNA glycosylase. Biochemistry 48, 11357–11369 (2009).
Hendershot, J.M., Wolfe, A.E. & O'Brien, P.J. Substitution of active site tyrosines with tryptophan alters the free energy for nucleotide flipping by human alkyladenine DNA glycosylase. Biochemistry 50, 1864–1874 (2011).
Bowman, B.R., Lee, S., Wang, S. & Verdine, G.L. Structure of Escherichia coli AlkA in complex with undamaged DNA. J. Biol. Chem. 285, 35783–35791 (2010).
Hollis, T., Ichikawa, Y. & Ellenberger, T. DNA bending and a flip-out mechanism for base excision by the helix-hairpin-helix DNA glycosylase, Escherichia coli AlkA. EMBO J. 19, 758–766 (2000).
Banerjee, A., Santos, W.L. & Verdine, G.L. Structure of a DNA glycosylase searching for lesions. Science 311, 1153–1157 (2006).
Qi, Y. et al. Encounter and extrusion of an intrahelical lesion by a DNA repair enzyme. Nature 462, 762–766 (2009).
Banerjee, A., Yang, W., Karplus, M. & Verdine, G.L. Structure of a repair enzyme interrogating undamaged DNA elucidates recognition of damaged DNA. Nature 434, 612–618 (2005).
Slupphaug, G. et al. A nucleotide-flipping mechanism from the structure of human uracil-DNA glycosylase bound to DNA. Nature 384, 87–92 (1996).
Cao, C., Jiang, Y.L., Stivers, J.T. & Song, F.H. Dynamic opening of DNA during the enzymatic search for a damaged base. Nat. Struct. Mol. Biol. 11, 1230–1236 (2004).
Parker, J.B. et al. Enzymatic capture of an extrahelical thymine in the search for uracil in DNA. Nature 449, 433–437 (2007).
Fromme, J.C., Banerjee, A. & Verdine, G.L. DNA glycosylase recognition and catalysis. Curr. Opin. Struct. Biol. 14, 43–49 (2004).
Friedman, J.I. & Stivers, J.T. Detection of damaged DNA bases by DNA glycosylase enzymes. Biochemistry 49, 4957–4967 (2010).
Huang, H., Chopra, R., Verdine, G.L. & Harrison, S.C. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science 282, 1669–1675 (1998).
Monsen, V.T. et al. Divergent β-hairpins determine double-strand versus single-strand substrate recognition of human AlkB-homologues 2 and 3. Nucleic Acids Res. 38, 6447–6455 (2010).
Chen, B., Liu, H., Sun, X. & Yang, C.G. Mechanistic insight into the recognition of single-stranded and double-stranded DNA substrates by ABH2 and ABH3. Mol. Biosyst. 6, 2143–2149 (2010).
Ma, A., Hu, J., Karplus, M. & Dinner, A.R. Implications of alternative substrate binding modes for catalysis by uracil-DNA glycosylase: an apparent discrepancy resolved. Biochemistry 45, 13687–13696 (2006).
Lu, L., Yi, C., Jian, X., Zheng, G. & He, C. Structure determination of DNA methylation lesions N1-meA and N3-meC in duplex DNA using a cross-linked protein-DNA system. Nucleic Acids Res. 38, 4415–4425 (2010).
Bowman, B.R., Lee, S., Wang, S. & Verdine, G.L. Structure of the E. coli DNA glycosylase AlkA bound to the ends of duplex DNA: a system for the structure determination of lesion-containing DNA. Structure 16, 1166–1174 (2008).
Cheng, X. & Blumenthal, R.M. Mammalian DNA methyltransferases: a structural perspective. Structure 16, 341–350 (2008).
Yu, B. et al. Crystal structures of catalytic complexes of the oxidative DNA/RNA repair enzyme AlkB. Nature 439, 879–884 (2006).
Yi, C. et al. Iron-catalysed oxidation intermediates captured in a DNA repair dioxygenase. Nature 468, 330–333 (2010).
Yu, B. & Hunt, J.F. Enzymological and structural studies of the mechanism of promiscuous substrate recognition by the oxidative DNA repair enzyme AlkB. Proc. Natl. Acad. Sci. USA 106, 14315–14320 (2009).
Leonard, G.A. et al. Guanine-1,N6-ethenoadenine base pairs in the crystal structure of d(CGCGAATT(epsilon dA)GCG). Biochemistry 33, 4755–4761 (1994).
Tahiliani, M. et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935 (2009).
Blainey, P.C., van Oijen, A.M., Banerjee, A., Verdine, G.L. & Xie, X.S. A base-excision DNA-repair protein finds intrahelical lesion bases by fast sliding in contact with DNA. Proc. Natl. Acad. Sci. USA 103, 5752–5757 (2006).
Blainey, P.C. et al. Nonspecifically bound proteins spin while diffusing along DNA. Nat. Struct. Mol. Biol. 16, 1224–1229 (2009).
Lin, Y. et al. Using the bias from flow to elucidate single DNA repair protein sliding and interactions with DNA. Biophys. J. 96, 1911–1917 (2009).
Hedglin, M. & O'Brien, P.J. Hopping enables a DNA repair glycosylase to search both strands and bypass a bound protein. ACS Chem. Biol. 5, 427–436 (2010).
Schonhoft, J.D. & Stivers, J.T. Timing facilitated site transfer of an enzyme on DNA. Nat. Chem. Biol. 8, 205–210 (2012).
Sun, Y., Friedman, J.I. & Stivers, J.T. Cosolute paramagnetic relaxation enhancements detect transient conformations of human uracil DNA glycosylase (hUNG). Biochemistry 50, 10724–10731 (2011).
Dango, S. et al. DNA unwinding by ASCC3 helicase is coupled to ALKBH3-dependent DNA alkylation repair and cancer cell proliferation. Mol. Cell 44, 373–384 (2011).
Sundheim, O. et al. Human ABH3 structure and key residues for oxidative demethylation to reverse DNA/RNA damage. EMBO J. 25, 3389–3397 (2006).
Krebs, C. et al. Rapid freeze-quench 57Fe Mossbauer spectroscopy: monitoring changes of an iron-containing active site during a biochemical reaction. Inorg. Chem. 44, 742–757 (2005).
Schofield, C.J. & Zhang, Z.H. Structural and mechanistic studies on 2-oxoglutarate-dependent oxygenases and related enzymes. Curr. Opin. Struct. Biol. 9, 722–731 (1999).
Costas, M., Mehn, M.P., Jensen, M.P. & Que, L. Dioxygen activation at mononuclear nonheme iron active sites:enzymes, models, and intermediates. Chem. Rev. 104, 939–986 (2004).
Liu, H., Llano, J. & Gauld, J.W.A. DFT study of nucleobase dealkylation by the DNA repair enzyme AlkB. J. Phys. Chem. B 113, 4887–4898 (2009).
Tubbs, J.L. et al. Flipping of alkylated DNA damage bridges base and nucleotide excision repair. Nature 459, 808–813 (2009).
Rubinson, E.H., Gowda, A.S., Spratt, T.E., Gold, B. & Eichman, B.F. An unprecedented nucleic acid capture mechanism for excision of DNA damage. Nature 468, 406–411 (2010).
Mishina, Y., Chen, L.X. & He, C. Preparation and characterization of the native iron(II)-containing DNA repair AlkB protein directly from Escherichia coli. J. Am. Chem. Soc. 126, 16930–16936 (2004).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Read, R.J. Pushing the boundaries of molecular replacement with maximum likelihood. Acta Crystallogr. D Biol. Crystallogr. 57, 1373–1382 (2001).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Acknowledgements
This study was supported by the US National Institutes of Health (GM071440 to C.H.), the Hundred Talent Program of the Chinese Academy of Sciences (to C.-G.Y.), the State Key Development Program of Basic Research of China (2009CB918502 to C.-G.Y.), the special grant for Stem Cell and Regenerative Medicine (XDA01040305 to C.-G.Y.), the National Science Foundation (MCB-0547854 to A.R.D.), Beamlines 23ID-B (General Medicine and Cancer Institutes Collaborative Access Team, GM/CA-CAT), 21ID-D (Life Sciences Collaborative Access Team, LS-CAT) and 24ID-E (The Northeastern Collaborative Access Team, NE-CAT) at the Advanced Photon Source at Argonne National Laboratory, the Shanghai Synchrotron Radiation Facility (BL17U1) and the US Department of Energy.
Author information
Authors and Affiliations
Contributions
C.Y., C.-G.Y. and C.H. designed the experiments. Experiments were conducted by C.Y., B.C., W.Z., G.J., L.Z. and C.J.L.; computational analyses were carried out by B.Q. and A.R.D. C.Y. and C.H. wrote the paper, and B.Q., A.R.D. and C.-G.Y. contributed to editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1, Supplementary Figures 1–5 and Supplementary Note (PDF 3819 kb)
Rights and permissions
About this article
Cite this article
Yi, C., Chen, B., Qi, B. et al. Duplex interrogation by a direct DNA repair protein in search of base damage. Nat Struct Mol Biol 19, 671–676 (2012). https://doi.org/10.1038/nsmb.2320
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2320
This article is cited by
-
Demethyltransferase AlkBH1 substrate diversity and relationship to human diseases
Molecular Biology Reports (2021)
-
RNA-modifying proteins as anticancer drug targets
Nature Reviews Drug Discovery (2018)
-
Kinetic gating mechanism of DNA damage recognition by Rad4/XPC
Nature Communications (2015)
-
A Comprehensive View of the Epigenetic Landscape Part I: DNA Methylation, Passive and Active DNA Demethylation Pathways and Histone Variants
Neurotoxicity Research (2015)
-
The complex structures of ALKBH2 mutants cross-linked to dsDNA reveal the conformational swing of β-hairpin
Science China Chemistry (2014)