Abstract
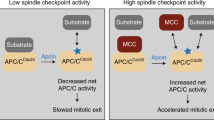
The anaphase-promoting complex/cyclosome (APC/C) bound to CDC20 (APC/CCDC20) initiates anaphase by ubiquitylating B-type cyclins and securin. During chromosome bi-orientation, CDC20 assembles with MAD2, BUBR1 and BUB3 into a mitotic checkpoint complex (MCC) that inhibits substrate recruitment to the APC/C. APC/C activation depends on MCC disassembly, which was proposed to require CDC20 autoubiquitylation. Here we characterize APC15, a human APC/C subunit related to yeast Mnd2. APC15 is located near APC/C's MCC binding site; it is required for APC/C-bound MCC (APC/CMCC)-dependent CDC20 autoubiquitylation and degradation and for timely anaphase initiation but is dispensable for substrate ubiquitylation by APC/CCDC20 and APC/CCDH1. Our results support the model wherein MCC is continuously assembled and disassembled to enable rapid activation of APC/CCDC20 and CDC20 autoubiquitylation promotes MCC disassembly. We propose that APC15 and Mnd2 negatively regulate APC/C coactivators and report generation of recombinant human APC/C.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Musacchio, A. Spindle assembly checkpoint: the third decade. Phil. Trans. R. Soc. Lond. B 366, 3595–3604 (2011).
Rieder, C.L., Cole, R.W., Khodjakov, A. & Sluder, G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 130, 941–948 (1995).
Stern, B.M. & Murray, A.W. Lack of tension at kinetochores activates the spindle checkpoint in budding yeast. Curr. Biol. 11, 1462–1467 (2001).
Fang, G., Yu, H. & Kirschner, M.W. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 12, 1871–1883 (1998).
Hwang, L.H. et al. Budding yeast Cdc20: a target of the spindle checkpoint. Science 279, 1041–1044 (1998).
Kim, S.H., Lin, D.P., Matsumoto, S., Kitazono, A. & Matsumoto, T. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science 279, 1045–1047 (1998).
Kramer, E.R., Gieffers, C., Holzl, G., Hengstschlager, M. & Peters, J.M. Activation of the human anaphase-promoting complex by proteins of the CDC20/Fizzy family. Curr. Biol. 8, 1207–1210 (1998).
Kramer, E.R., Scheuringer, N., Podtelejnikov, A.V., Mann, M. & Peters, J.M. Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol. Biol. Cell 11, 1555–1569 (2000).
Peters, J.M. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell 9, 931–943 (2002).
Dube, P. et al. Localization of the coactivator Cdh1 and the cullin subunit Apc2 in a cryo-electron microscopy model of vertebrate APC/C. Mol. Cell 20, 867–879 (2005).
Herzog, F. et al. Structure of the anaphase-promoting complex/cyclosome interacting with a mitotic checkpoint complex. Science 323, 1477–1481 (2009).
Buschhorn, B.A. et al. Substrate binding on the APC/C occurs between the coactivator Cdh1 and the processivity factor Doc1. Nat. Struct. Mol. Biol. 18, 6–13 (2011).
Gmachl, M., Gieffers, C., Podtelejnikov, A.V., Mann, M. & Peters, J.M. The RING-H2 finger protein APC11 and the E2 enzyme UBC4 are sufficient to ubiquitinate substrates of the anaphase-promoting complex. Proc. Natl. Acad. Sci. USA 97, 8973–8978 (2000).
Leverson, J.D. et al. The APC11 RING-H2 finger mediates E2-dependent ubiquitination. Mol. Biol. Cell 11, 2315–2325 (2000).
Tang, Z. et al. APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Mol. Biol. Cell 12, 3839–3851 (2001).
Glotzer, M., Murray, A.W. & Kirschner, M.W. Cyclin is degraded by the ubiquitin pathway. Nature 349, 132–138 (1991).
Pfleger, C.M. & Kirschner, M.W. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 14, 655–665 (2000).
Kraft, C., Vodermaier, H.C., Maurer-Stroh, S., Eisenhaber, F. & Peters, J.M. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol. Cell 18, 543–553 (2005).
da Fonseca, P.C. et al. Structures of APC/C(Cdh1) with substrates identify Cdh1 and Apc10 as the D-box co-receptor. Nature 470, 274–278 (2011).
Chao, W.C., Kulkarni, K., Zhang, Z., Kong, E.H. & Barford, D. Structure of the mitotic checkpoint complex. Nature 484, 208–213 (2012).
Sudakin, V., Chan, G.K. & Yen, T.J. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 154, 925–936 (2001).
Luo, X., Tang, Z., Rizo, J. & Yu, H. The Mad2 spindle checkpoint protein undergoes similar major conformational changes upon binding to either Mad1 or Cdc20. Mol. Cell 9, 59–71 (2002).
Sironi, L. et al. Crystal structure of the tetrameric Mad1-Mad2 core complex: implications of a 'safety belt' binding mechanism for the spindle checkpoint. EMBO J. 21, 2496–2506 (2002).
De Antoni, A. et al. The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr. Biol. 15, 214–225 (2005).
Mapelli, M., Massimiliano, L., Santaguida, S. & Musacchio, A. The Mad2 conformational dimer: structure and implications for the spindle assembly checkpoint. Cell 131, 730–743 (2007).
Burton, J.L. & Solomon, M.J. Mad3p, a pseudosubstrate inhibitor of APCCdc20 in the spindle assembly checkpoint. Genes Dev. 21, 655–667 (2007).
Reddy, S.K., Rape, M., Margansky, W.A. & Kirschner, M.W. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature 446, 921–925 (2007).
Miniowitz-Shemtov, S., Teichner, A., Sitry-Shevah, D. & Hershko, A. ATP is required for the release of the anaphase-promoting complex/cyclosome from inhibition by the mitotic checkpoint. Proc. Natl. Acad. Sci. USA 107, 5351–5356 (2010).
Varetti, G., Guida, C., Santaguida, S., Chiroli, E. & Musacchio, A. Homeostatic control of mitotic arrest. Mol. Cell 44, 710–720 (2011).
Nilsson, J., Yekezare, M., Minshull, J. & Pines, J. The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat. Cell Biol. 10, 1411–1420 (2008).
Gao, Y.F. et al. Cdk1-phosphorylated CUEDC2 promotes spindle checkpoint inactivation and chromosomal instability. Nat. Cell Biol. 13, 924–933 (2011).
Jia, L. et al. Defining pathways of spindle checkpoint silencing: functional redundancy between Cdc20 ubiquitination and p31(comet). Mol. Biol. Cell 22, 4227–4235 (2011).
Teichner, A. et al. p31comet promotes disassembly of the mitotic checkpoint complex in an ATP-dependent process. Proc. Natl. Acad. Sci. USA 108, 3187–3192 (2011).
Westhorpe, F.G., Tighe, A., Lara-Gonzalez, P. & Taylor, S.S. p31comet-mediated extraction of Mad2 from the MCC promotes efficient mitotic exit. J. Cell Sci. 124, 3905–3916 (2011).
Miniowitz-Shemtov, S. et al. Role of phosphorylation of Cdc20 in p31(comet)-stimulated disassembly of the mitotic checkpoint complex. Proc. Natl. Acad. Sci. USA 109, 8056–8060 (2012).
Xia, G. et al. Conformation-specific binding of p31(comet) antagonizes the function of Mad2 in the spindle checkpoint. EMBO J. 23, 3133–3143 (2004).
Yang, M. et al. p31comet blocks Mad2 activation through structural mimicry. Cell 131, 744–755 (2007).
Kittler, R. et al. Genome-scale RNAi profiling of cell division in human tissue culture cells. Nat. Cell Biol. 9, 1401–1412 (2007).
Hubner, N.C. et al. Quantitative proteomics combined with BAC TransgeneOmics reveals in vivo protein interactions. J. Cell Biol. 189, 739–754 (2010).
Mansfeld, J., Collin, P., Collins, M.O., Choudhary, J.S. & Pines, J. APC15 drives the turnover of MCC-CDC20 to make the spindle assembly checkpoint responsive to kinetochore attachment. Nat. Cell Biol. 13, 1234–1243 (2011).
Hutchins, J.R. et al. Systematic analysis of human protein complexes identifies chromosome segregation proteins. Science 328, 593–599 (2010).
Kops, G.J. et al. APC16 is a conserved subunit of the anaphase-promoting complex/cyclosome. J. Cell Sci. 123, 1623–1633 (2010).
Cheeseman, I.M. & Desai, A. A combined approach for the localization and tandem affinity purification of protein complexes from metazoans. Sci. STKE 2005, pl1 (2005).
Poser, I. et al. BAC TransgeneOmics: a high-throughput method for exploration of protein function in mammals. Nat. Methods 5, 409–415 (2008).
Zachariae, W. et al. Mass spectrometric analysis of the anaphase-promoting complex from yeast: identification of a subunit related to cullins. Science 279, 1216–1219 (1998).
Hall, M.C., Torres, M.P., Schroeder, G.K. & Borchers, C.H. Mnd2 and Swm1 are core subunits of the Saccharomyces cerevisiae anaphase-promoting complex. J. Biol. Chem. 278, 16698–16705 (2003).
Schreiber, A. et al. Structural basis for the subunit assembly of the anaphase-promoting complex. Nature 470, 227–232 (2011).
Visconti, R., Palazzo, L. & Grieco, D. Requirement for proteolysis in spindle assembly checkpoint silencing. Cell Cycle 9, 564–569 (2010).
Zeng, X. et al. Pharmacologic inhibition of the anaphase-promoting complex induces a spindle checkpoint-dependent mitotic arrest in the absence of spindle damage. Cancer Cell 18, 382–395 (2010).
Ma, H.T. & Poon, R.Y. Orderly inactivation of the key checkpoint protein mitotic arrest deficient 2 (MAD2) during mitotic progression. J. Biol. Chem. 286, 13052–13059 (2011).
Foe, I.T. et al. Ubiquitination of Cdc20 by the APC occurs through an intramolecular mechanism. Curr. Biol. 21, 1870–1877 (2011).
Oelschlaegel, T. et al. The yeast APC/C subunit Mnd2 prevents premature sister chromatid separation triggered by the meiosis-specific APC/C-Ama1. Cell 120, 773–788 (2005).
Penkner, A.M., Prinz, S., Ferscha, S. & Klein, F. Mnd2, an essential antagonist of the anaphase-promoting complex during meiotic prophase. Cell 120, 789–801 (2005).
Foster, S.A. & Morgan, D.O. The APC/C subunit Mnd2/Apc15 promotes Cdc20 autoubiquitination and spindle assembly checkpoint inactivation. Mol. Cell published online, doi:10.1016/j.molcel.2012.07.031 (30 August 2012).
Elbashir, S.M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
Kastner, B. et al. GraFix: sample preparation for single-particle electron cryomicroscopy. Nat. Methods 5, 53–55 (2008).
Williamson, A., Jin, L. & Rape, M. Preparation of synchronized human cell extracts to study ubiquitination and degradation. Methods Mol. Biol. 545, 301–312 (2009).
Acknowledgements
Research in the laboratory of J.-M.P is supported by Boehringer Ingelheim, the Vienna Science and Technology Fund (WWTF LS09-13), the Laura Bassi Center for Optimized Structural Studies (FFG 822736) and the Austrian Science Fund (FWF special research program SFB F34 'Chromosome Dynamics', grant W1221 'DK: Structure and Interaction of Biological Macromolecules' and Wittgenstein award Z196-B20). Research in the laboratories of J.-M.P. and A.A.H. is supported by the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 241548 (MitoSys). Research in the laboratory of B.A.S. is supported by the American Lebanese Syrian Associated Charities/St. Jude and the Howard Hughes Medical Institute. Research in the laboratory of H. Stark is supported by the German Research Foundation (DFG) under grant agreement SFB860. Research in the laboratory of K.M. is supported by European Community's Seventh Framework Programme under grant agreement no. 262067 (PRIME-XS). Y.T. was supported by the Japan Society for the Promotion of Science (Postdoctoral Fellowship for Research Abroad). N.G.B. is supported as a Fellow of the Jane Coffin Childs Memorial Fund for Medical Research.
Author information
Authors and Affiliations
Contributions
B.A.S. and J.-M.P. planned and supervised the project. K.U., B.T.D., H. Schutz, R.L., G.P., Y.T., M.A.J., N.G.B. and I.P. designed the experiments. K.U. performed experiments on APC15 function. B.T.D. and N.G.B. performed experiments on recombinant human APC/C. M.A.J. and G.P. established protocols for coactivator protein purification. H. Schutz performed experiments on APC15 being an APC/C core subunit. R.L. performed IFM experiments. G.P. performed antibody labeling and contributed to EM experiments. Y.T. performed time-lapse microscopy. I.P. and A.A.H. created LAP-tagged APC15. M.N. performed APC15 homology searches. K.M. performed mass spectrometry. H. Stark performed EM experiments, calculated 3D EM structures and analyzed APC15 antibody labeling. K.U., R.L., G.P., B.T.D., N.G.B., B.A.S. and J.-M.P. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Note (PDF 1547 kb)
Rights and permissions
About this article
Cite this article
Uzunova, K., Dye, B., Schutz, H. et al. APC15 mediates CDC20 autoubiquitylation by APC/CMCC and disassembly of the mitotic checkpoint complex. Nat Struct Mol Biol 19, 1116–1123 (2012). https://doi.org/10.1038/nsmb.2412
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2412
This article is cited by
-
Principles and dynamics of spindle assembly checkpoint signalling
Nature Reviews Molecular Cell Biology (2023)
-
Homozygous variants in CDC23 cause female infertility characterized by oocyte maturation defects
Human Genetics (2023)
-
Mitotic phosphorylation of tumor suppressor DAB2IP maintains spindle assembly checkpoint and chromosomal stability through activating PLK1-Mps1 signal pathway and stabilizing mitotic checkpoint complex
Oncogene (2022)
-
Assembly defects of human tRNA splicing endonuclease contribute to impaired pre-tRNA processing in pontocerebellar hypoplasia
Nature Communications (2021)
-
Polyanions provide selective control of APC/C interactions with the activator subunit
Nature Communications (2019)