Abstract
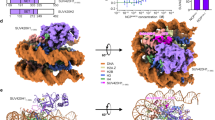
ATR-X (alpha-thalassemia/mental retardation, X-linked) syndrome is a human congenital disorder that causes severe intellectual disabilities. Mutations in the ATRX gene, which encodes an ATP-dependent chromatin-remodeler, are responsible for the syndrome. Approximately 50% of the missense mutations in affected persons are clustered in a cysteine-rich domain termed ADD (ATRX-DNMT3-DNMT3L, ADDATRX), whose function has remained elusive. Here we identify ADDATRX as a previously unknown histone H3–binding module, whose binding is promoted by lysine 9 trimethylation (H3K9me3) but inhibited by lysine 4 trimethylation (H3K4me3). The cocrystal structure of ADDATRX bound to H31–15K9me3 peptide reveals an atypical composite H3K9me3-binding pocket, which is distinct from the conventional trimethyllysine-binding aromatic cage. Notably, H3K9me3-pocket mutants and ATR-X syndrome mutants are defective in both H3K9me3 binding and localization at pericentromeric heterochromatin; thus, we have discovered a unique histone-recognition mechanism underlying the ATR-X etiology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Li, H. et al. Molecular basis for site-specific read-out of histone H3K4me3 by the BPTF PHD finger of NURF. Nature 442, 91–95 (2006).
Wysocka, J. et al. A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature 442, 86–90 (2006).
Shi, X. et al. ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature 442, 96–99 (2006).
Peña, P.V. et al. Molecular mechanism of histone H3K4me3 recognition by plant homeodomain of ING2. Nature 442, 100–103 (2006).
Bannister, A.J. et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410, 120–124 (2001).
Lachner, M., O'Carroll, D., Rea, S., Mechtler, K. & Jenuwein, T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410, 116–120 (2001).
Berger, S.L. The complex language of chromatin regulation during transcription. Nature 447, 407–412 (2007).
van Bokhoven, H. & Kramer, J.M. Disruption of the epigenetic code: an emerging mechanism in mental retardation. Neurobiol. Dis. 39, 3–12 (2010).
Iwase, S. & Shi, Y. Histone and DNA modifications in mental retardation. Prog. Drug Res. 67, 147–173 (2011).
Ropers, H.H. & Hamel, B.C. X-linked mental retardation. Nat. Rev. Genet. 6, 46–57 (2005).
Gibbons, R.J., Picketts, D.J., Villard, L. & Higgs, D.R. Mutations in a putative global transcriptional regulator cause X-linked mental retardation with alpha-thalassemia (ATR-X syndrome). Cell 80, 837–845 (1995).
Gibbons, R. Alpha thalassaemia-mental retardation, X linked. Orphanet J. Rare Dis. 1, 15 (2006).
Gibbons, R.J. et al. Mutations in the chromatin-associated protein ATRX. Hum. Mutat. 29, 796–802 (2008).
Tang, J. et al. A novel transcription regulatory complex containing death domain-associated protein and the ATR-X syndrome protein. J. Biol. Chem. 279, 20369–20377 (2004).
Ooi, S.K. et al. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature 448, 714–717 (2007).
Otani, J. et al. Structural basis for recognition of H3K4 methylation status by the DNA methyltransferase 3A ATRX-DNMT3-DNMT3L domain. EMBO Rep. 10, 1235–1241 (2009).
Zhang, Y. et al. Chromatin methylation activity of Dnmt3a and Dnmt3a/3L is guided by interaction of the ADD domain with the histone H3 tail. Nucleic Acids Res. 38, 4246–4253 (2010).
Goldberg, A.D. et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell 140, 678–691 (2010).
McDowell, T.L. et al. Localization of a putative transcriptional regulator (ATRX) at pericentromeric heterochromatin and the short arms of acrocentric chromosomes. Proc. Natl. Acad. Sci. USA 96, 13983–13988 (1999).
Allshire, R.C. & Karpen, G.H. Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nat. Rev. Genet. 9, 923–937 (2008).
Pidoux, A.L. & Allshire, R.C. The role of heterochromatin in centromere function. Phil. Trans. R. Soc. Lond. B 360, 569–579 (2005).
Sullivan, B.A. & Karpen, G.H. Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nat. Struct. Mol. Biol. 11, 1076–1083 (2004).
Martens, J.H. et al. The profile of repeat-associated histone lysine methylation states in the mouse epigenome. EMBO J. 24, 800–812 (2005).
Rea, S. et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406, 593–599 (2000).
O'Carroll, D. et al. Isolation and characterization of Suv39h2, a second histone H3 methyltransferase gene that displays testis-specific expression. Mol. Cell. Biol. 20, 9423–9433 (2000).
Rice, J.C. et al. Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Mol. Cell 12, 1591–1598 (2003).
Nan, X. et al. Interaction between chromatin proteins MECP2 and ATRX is disrupted by mutations that cause inherited mental retardation. Proc. Natl. Acad. Sci. USA 104, 2709–2714 (2007).
Lechner, M.S., Schultz, D.C., Negorev, D., Maul, G.G. & Rauscher, F.J. III. The mammalian heterochromatin protein 1 binds diverse nuclear proteins through a common motif that targets the chromoshadow domain. Biochem. Biophys. Res. Commun. 331, 929–937 (2005).
Bérubé, N.G., Smeenk, C.A. & Picketts, D.J. Cell cycle-dependent phosphorylation of the ATRX protein correlates with changes in nuclear matrix and chromatin association. Hum. Mol. Genet. 9, 539–547 (2000).
Xue, Y. et al. The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc. Natl. Acad. Sci. USA 100, 10635–10640 (2003).
Taverna, S.D., Li, H., Ruthenburg, A.J., Allis, C.D. & Patel, D.J. How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. Nat. Struct. Mol. Biol. 14, 1025–1040 (2007).
Maurer-Stroh, S. et al. The Tudor domain 'Royal Family': Tudor, plant Agenet, Chromo, PWWP and MBT domains. Trends Biochem. Sci. 28, 69–74 (2003).
Collins, R.E. et al. The ankyrin repeats of G9a and GLP histone methyltransferases are mono- and dimethyllysine binding modules. Nat. Struct. Mol. Biol. 15, 245–250 (2008).
Jacobs, S.A. et al. Specificity of the HP1 chromo domain for the methylated N-terminus of histone H3. EMBO J. 20, 5232–5241 (2001).
Schalch, T. et al. High-affinity binding of Chp1 chromodomain to K9 methylated histone H3 is required to establish centromeric heterochromatin. Mol. Cell 34, 36–46 (2009).
Fischle, W. et al. Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by Polycomb and HP1 chromodomains. Genes Dev. 17, 1870–1881 (2003).
Argentaro, A. et al. Structural consequences of disease-causing mutations in the ATRX-DNMT3-DNMT3L (ADD) domain of the chromatin-associated protein ATRX. Proc. Natl. Acad. Sci. USA 104, 11939–11944 (2007).
Allshire, R.C., Nimmo, E.R., Ekwall, K., Javerzat, J.P. & Cranston, G. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 9, 218–233 (1995).
Guenatri, M., Bailly, D., Maison, C. & Almouzni, G. Mouse centric and pericentric satellite repeats form distinct functional heterochromatin. J. Cell Biol. 166, 493–505 (2004).
Ritchie, K. et al. Loss of ATRX leads to chromosome cohesion and congression defects. J. Cell Biol. 180, 315–324 (2008).
Bérubé, N.G. et al. The chromatin-remodeling protein ATRX is critical for neuronal survival during corticogenesis. J. Clin. Invest. 115, 258–267 (2005).
Chen, T. et al. Complete inactivation of DNMT1 leads to mitotic catastrophe in human cancer cells. Nat. Genet. 39, 391–396 (2007).
Ha, G.H. et al. Mitotic catastrophe is the predominant response to histone acetyltransferase depletion. Cell Death Differ. 16, 483–497 (2009).
Wong, L.H. et al. ATRX interacts with H3.3 in maintaining telomere structural integrity in pluripotent embryonic stem cells. Genome Res. 20, 351–360 (2010).
Lewis, P.W., Elsaesser, S.J., Noh, K.M., Stadler, S.C. & Allis, C.D. Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc. Natl. Acad. Sci. USA 107, 14075–14080 (2010).
Drané, P., Ouararhni, K., Depaux, A., Shuaib, M. & Hamiche, A. The death-associated protein DAXX is a novel histone chaperone involved in the replication-independent deposition of H3.3. Genes Dev. 24, 1253–1265 (2010).
Zeng, L. et al. Mechanism and regulation of acetylated histone binding by the tandem PHD finger of DPF3b. Nature 466, 258–262 (2010).
Tsai, W.-W. TRIM24 links recognition of a non-canonical histone signature to breast cancer. Nature 468, 927–932 (2010).
Schnitzler, G.R. Isolation of histones and nucleosome cores from mammalian cells. Curr. Protoc. Mol. Biol. 21, 21.5.1–21.5.12 (2001).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Vagin, A. & Teplyakov, A. MOLREP: an automated program for molecular replacement. J. Appl. Crystallogr. 30, 1022–1025 (1997).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Iwase, S. et al. The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell 128, 1077–1088 (2007).
Acknowledgements
The authors are grateful to the staffs of beamlines X-29 at the Brookhaven National Laboratory, 24IDC at the Advanced Photon Source and 3W1A at the Beijing Synchrotron Radiation Facility (BSRF) for their assistance in the synchrotron data collection, with special thanks to Y. Dong of BSRF. We thank A. Vaquero (Bellvitge Institute for Biomedical Research) and S. Minucci (European Institute of Oncology) for providing the Suv39h1 and Suv39h2 double knockout cells and SUV39H1 expression vectors. Anti-MeCP2 antiserum was a generous gift from M. Greenberg (Harvard Medical School, HMS). We are grateful to A. Ruthenburg (Rockefeller University) for providing histone H3 peptides and to L. Liang (Memorial Sloan-Kettering Cancer Center), Y.Y. Chen (Institute of Biophysics, Chinese Academy of Sciences) and M. Geigges (Children's Hospital Boston) for experimental help. We express great appreciation to C. Richardson, M. Takahashi at HMS and individual members of the Shi, Li and Patel laboratories for helpful discussions. S.I. was a Jane Coffin Childs Memorial Fund Postdoctoral Fellowship Award recipient and is currently supported by the Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad. This work was supported by grants GM071004 and GM058012 from the US National Institutes of Health (Y.S.) and by the Starr Foundation, the Leukemia and Lymphoma Society, the Abby Rockefeller Mauze Trust and the Maloris Foundation (D.J. Patel); the Canadian Institutes of Health Research (D.J. Picketts); the Major State Basic Research Development Program in China (grant # 2011CB965300, H.L.) and by Tsinghua University 985 Phase II funds (H.L.).
Author information
Authors and Affiliations
Contributions
S.I., H.L., D.J. Patel and Y.S. participated in the experimental design, contributed to the concept and wrote the paper. S.I. did pulldown assays, SPR assays and localization analysis. B.X. crystallized the ADDATRX–H3K9me3 complex and participated in calorimetric assays. H.L. carried out crystallographic and calorimetric studies. P.W.L., T.R. and J.C.C. carried out or helped with pulldown assays. S.G. helped with measuring SPR. C.D.A. supervised pulldown assays. D.J. Picketts provided important reagents and participated in manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
Y.S. is a cofounder of Constellation Pharmaceuticals. D.J. Patel is on the Epigenetics Advisory Board of Epinova-Glaxo. The remaining authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9, Supplementary Table 1 and Supplementary Methods (PDF 13312 kb)
Rights and permissions
About this article
Cite this article
Iwase, S., Xiang, B., Ghosh, S. et al. ATRX ADD domain links an atypical histone methylation recognition mechanism to human mental-retardation syndrome. Nat Struct Mol Biol 18, 769–776 (2011). https://doi.org/10.1038/nsmb.2062
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2062
This article is cited by
-
DNMT3B PWWP mutations cause hypermethylation of heterochromatin
EMBO Reports (2024)
-
TRIM28 inhibits alternative lengthening of telomere phenotypes by protecting SETDB1 from degradation
Cell & Bioscience (2021)
-
ATRX promotes heterochromatin formation to protect cells from G-quadruplex DNA-mediated stress
Nature Communications (2021)
-
Deletion of the XIST promoter from the human inactive X chromosome compromises polycomb heterochromatin maintenance
Chromosoma (2021)
-
Neuroblastoma and the epigenome
Cancer and Metastasis Reviews (2021)