Abstract
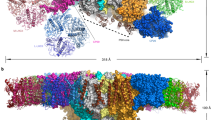
CP29, one of the minor light-harvesting complexes of higher-plant photosystem II, absorbs and transfers solar energy for photosynthesis and also has important roles in photoprotection. We have solved the crystal structure of spinach CP29 at 2.80-Å resolution. Each CP29 monomer contains 13 chlorophyll and 3 carotenoid molecules, which differs considerably from the major light-harvesting complex LHCII and the previously proposed CP29 model. The 13 chlorophyll-binding sites are assigned as eight chlorophyll a sites, four chlorophyll b and one putative mixed site occupied by both chlorophylls a and b. Based on the present X-ray structure, an integrated pigment network in CP29 is constructed. Two special clusters of pigment molecules, namely a615–a611–a612–Lut and Vio(Zea)–a603–a609, have been identified and might function as potential energy-quenching centers and as the exit or entrance in energy-transfer pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Caffarri, S., Kouril, R., Kereiche, S., Boekema, E.J. & Croce, R. Functional architecture of higher plant photosystem II supercomplexes. EMBO J. 28, 3052–3063 (2009).
Horton, P., Ruban, A.V. & Walters, R.G. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 655–684 (1996).
Müller, P., Li, X.P. & Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 125, 1558–1566 (2001).
Liu, Z. et al. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428, 287–292 (2004).
Standfuss, J., Terwisscha van Scheltinga, A.C., Lamborghini, M. & Kuhlbrandt, W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. EMBO J. 24, 919–928 (2005).
van Oort, B. et al. Effect of antenna-depletion in Photosystem II on excitation energy transfer in Arabidopsis thaliana. Biophys. J. 98, 922–931 (2010).
Ahn, T.K. et al. Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein. Science 320, 794–797 (2008).
Avenson, T.J. et al. Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. J. Biol. Chem. 283, 3550–3558 (2008).
Cheng, Y.C. et al. Kinetic modeling of charge-transfer quenching in the CP29 minor complex. J. Phys. Chem. B 112, 13418–13423 (2008).
Caffarri, S., Passarini, F., Bassi, R. & Croce, R. A specific binding site for neoxanthin in the monomeric antenna proteins CP26 and CP29 of Photosystem II. FEBS Lett. 581, 4704–4710 (2007).
Bassi, R., Croce, R., Cugini, D. & Sandona, D. Mutational analysis of a higher plant antenna protein provides identification of chromophores bound into multiple sites. Proc. Natl. Acad. Sci. USA 96, 10056–10061 (1999).
Gastaldelli, M., Canino, G., Croce, R. & Bassi, R. Xanthophyll binding sites of the CP29 (Lhcb4) subunit of higher plant photosystem II investigated by domain swapping and mutation analysis. J. Biol. Chem. 278, 19190–19198 (2003).
Kavalenka, A.A. et al. Site-directed spin-labeling study of the light-harvesting complex CP29. Biophys. J. 96, 3620–3628 (2009).
Pascal, A. et al. Spectroscopic characterization of the spinach Lhcb4 protein (CP29), a minor light-harvesting complex of photosystem II. Eur. J. Biochem. 262, 817–823 (1999).
Ruban, A.V., Lee, P.J., Wentworth, M., Young, A.J. & Horton, P. Determination of the stoichiometry and strength of binding of xanthophylls to the photosystem II light harvesting complexes. J. Biol. Chem. 274, 10458–10465 (1999).
Das, S.K. & Frank, H.A. Pigment compositions, spectral properties, and energy transfer efficiencies between the xanthophylls and chlorophylls in the major and minor pigment–protein complexes of photosystem II. Biochemistry 41, 13087–13095 (2002).
Balaban, T.S. et al. Preferential pathways for light-trapping involving β-ligated chlorophylls. Biochim. Biophys. Acta 1787, 1254–1265 (2009).
Georgakopoulou, S. et al. Understanding the changes in the circular dichroism of light harvesting complex II upon varying its pigment composition and organization. Biochemistry 46, 4745–4754 (2007).
Gradinaru, C.C. et al. Ultrafast evolution of the excited states in the chlorophyll a/b complex CP29 from green plants studied by energy-selective pump-probe spectroscopy. Biochemistry 37, 1143–1149 (1998).
Cinque, G., Croce, R., Holzwarth, A. & Bassi, R. Energy transfer among CP29 chlorophylls: calculated Forster rates and experimental transient absorption at room temperature. Biophys. J. 79, 1706–1717 (2000).
Salverda, J.M. et al. Energy transfer in light-harvesting complexes LHCII and CP29 of spinach studied with three pulse echo peak shift and transient grating. Biophys. J. 84, 450–465 (2003).
Croce, R., Muller, M.G., Bassi, R. & Holzwarth, A.R. Chlorophyll b to chlorophyll a energy transfer kinetics in the CP29 antenna complex: a comparative femtosecond absorption study between native and reconstituted proteins. Biophys. J. 84, 2508–2516 (2003).
Croce, R., Muller, M.G., Caffarri, S., Bassi, R. & Holzwarth, A.R. Energy transfer pathways in the minor antenna complex CP29 of photosystem II: a femtosecond study of carotenoid to chlorophyll transfer on mutant and WT complexes. Biophys. J. 84, 2517–2532 (2003).
Dreuw, A., Fleming, G.R. & Head-Gordon, M. Chlorophyll fluorescence quenching by xanthophylls. Phys. Chem. Chem. Phys. 5, 3247–3256 (2003).
Gradinaru, C.C., van Stokkum, I.H.M., Pascal, A.A., van Grondelle, R. & van Amerongen, H. Identifying the pathways of energy transfer between carotenoids and chlorophylls in LHCII and CP29. A multicolor, femtosecond pump-probe study. J. Phys. Chem. B 104, 9330–9342 (2000).
Schödel, R., Irrgang, K.D., Voigt, J. & Renger, G. Quenching of chlorophyll fluorescence by triplets in solubilized light-harvesting complex II (LHCII). Biophys. J. 76, 2238–2248 (1999).
Mozzo, M., Dall'Osto, L., Hienerwadel, R., Bassi, R. & Croce, R. Photoprotection in the antenna complexes of photosystem II: role of individual xanthophylls in chlorophyll triplet quenching. J. Biol. Chem. 283, 6184–6192 (2008).
Dexter, D.L. A theory of sensitized luminescence in solids. J. Chem. Phys. 21, 836–850 (1953).
Damjanović, A., Ritz, T. & Schulten, K. Energy transfer between carotenoids and bacteriochlorophylls in light-harvesting complex II of purple bacteria. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics 59, 3293–3311 (1999).
Pascal, A.A. et al. Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature 436, 134–137 (2005).
Ruban, A.V. et al. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450, 575–578 (2007).
Mozzo, M., Passarini, F., Bassi, R., van Amerongen, H. & Croce, R. Photoprotection in higher plants: the putative quenching site is conserved in all outer light-harvesting complexes of Photosystem II. Biochim. Biophys. Acta 1777, 1263–1267 (2008).
Demmig, B., Winter, K., Kruger, A. & Czygan, F.C. Photoinhibition and zeaxanthin formation in intact leaves: a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 84, 218–224 (1987).
Gilmore, A.M. & Yamamoto, H.Y. Zeaxanthin formation and energy-dependent fluorescence quenching in pea chloroplasts under artificially mediated linear and cyclic electron transport. Plant Physiol. 96, 635–643 (1991).
Niyogi, K.K., Grossman, A.R. & Bjorkman, O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10, 1121–1134 (1998).
Ruban, A.V., Young, A.J., Pascal, A.A. & Horton, P. The effects of illumination on the xanthophyll composition of the Photosystem II light-harvesting complexes of spinach thylakoid membranes. Plant Physiol. 104, 227–234 (1994).
Morosinotto, T., Baronio, R. & Bassi, R. Dynamics of chromophore binding to Lhc proteins in vivo and in vitro during operation of the xanthophyll cycle. J. Biol. Chem. 277, 36913–36920 (2002).
Farber, A., Young, A.J., Ruban, A.V., Horton, P. & Jahns, P. Dynamics of xanthophyll-cycle activity in different antenna subcomplexes in the photosynthetic membranes of higher plants (the relationship between zeaxanthin conversion and nonphotochemical fluorescence quenching). Plant Physiol. 115, 1609–1618 (1997).
Pesaresi, P., Sandona, D., Giuffra, E. & Bassi, R. A single point mutation (E166Q) prevents dicyclohexylcarbodiimide binding to the photosystem II subunit CP29. FEBS Lett. 402, 151–156 (1997).
Bassi, R. & Caffarri, S. Lhc proteins and the regulation of photosynthetic light harvesting function by xanthophylls. Photosynth. Res. 64, 243–256 (2000).
Hager, A. & Holocher, K. Localization of the xanthophyll-cycle enzyme violaxanthin de-epoxidase within the thylakoid lumen and abolition of its mobility by a (light-dependent) pH decrease. Planta 192, 581–589 (1994).
Arnoux, P., Morosinotto, T., Saga, G., Bassi, R. & Pignol, D. A structural basis for the pH-dependent xanthophyll cycle in Arabidopsis thaliana. Plant Cell 21, 2036–2044 (2009).
Horton, P., Ruban, A.V. & Wentworth, M. Allosteric regulation of the light-harvesting system of photosystem II. Phil. Trans. R. Soc. Lond. B 355, 1361–1370 (2000).
Frank, H.A. et al. Photophysics of the carotenoids associated with the xanthophyll cycle in photosynthesis. Photosynth. Res. 41, 389–395 (1994).
Holt, N.E. et al. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307, 433–436 (2005).
Amarie, S. et al. Properties of zeaxanthin and its radical cation bound to the minor light-harvesting complexes CP24, CP26 and CP29. Biochim. Biophys. Acta 1787, 747–752 (2009).
Berera, R. et al. A simple artificial light-harvesting dyad as a model for excess energy dissipation in oxygenic photosynthesis. Proc. Natl. Acad. Sci. USA 103, 5343–5348 (2006).
Teardo, E. et al. Evidences for interaction of PsbS with photosynthetic complexes in maize thylakoids. Biochim. Biophys. Acta 1767, 703–711 (2007).
Li, X.P. et al. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403, 391–395 (2000).
Emanuelsson, O., Nielsen, H. & von Heijne, G. ChloroP, a neural network–based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci. 8, 978–984 (1999).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Collaborative Computing Project. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Strong, M. et al. Toward the structural genomics of complexes: crystal structure of a PE/PPE protein complex from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 103, 8060–8065 (2006).
Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Adams, P.D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 (2002).
Terwilliger, T.C. Maximum-likelihood density modification. Acta Crystallogr. D Biol. Crystallogr. 56, 965–972 (2000).
McCoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Brünger, A.T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998).
Laskowski, R.A., MacArthur, M.W., Moss, D.S. & Thornton, J.M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 (1993).
Acknowledgements
We thank D.C. Liang, X.C. Gu, R. Bassi, N. Isaacs and T. Jiang for discussions and the staffs at the Shanghai Synchrotron Radiation Facility, the Beijing Synchrotron Radiation Facility, SPring8 and the Photo Factory for technical support with crystal screening and data collection. This work was supported by National Natural Science Foundation of China grants 30530210, 30721003 and 31021062 (W.R.C.), 973 Project grants 2006CB806505, 2006CB911001 and 2011CBA00902 (W.R.C.) and Knowledge Innovation Program of the Chinese Academy of Sciences grant KSCX2-YW-R-123 (W.R.C.).
Author information
Authors and Affiliations
Contributions
X.W.P. did the purification, crystallization, data collection and processing, structure determination and structural analysis. M.L. assisted in data collection, structure analysis and manuscript preparation. T.W. did the protein sequence determination. L.F.W. assisted in data collection and structure determination. C.J.J., Z.Q.H., X.L.Z. and J.P.Z. assisted in sample isolation and purification. W.R.C. supervised the project and analyzed the structure. X.W.P. and W.R.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1–3 (PDF 3192 kb)
Rights and permissions
About this article
Cite this article
Pan, X., Li, M., Wan, T. et al. Structural insights into energy regulation of light-harvesting complex CP29 from spinach. Nat Struct Mol Biol 18, 309–315 (2011). https://doi.org/10.1038/nsmb.2008
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2008