Abstract
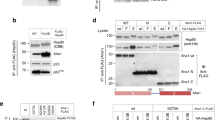
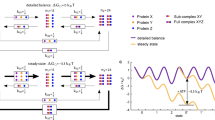
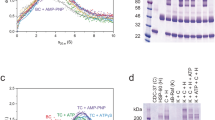
The chaperone cycle of heat shock protein-90 (Hsp90) involves progression through defined complexes with different cochaperones. It is still enigmatic how the exchange of cochaperones is regulated. The first cochaperone entering the cycle is the Hsp90 ATPase inhibitor Sti1 (Hop in human), which later is replaced by a prolyl isomerase (PPIase) and p23. We found, unexpectedly, that one Sti1 molecule is sufficient to completely inhibit the ATPase of the Hsp90 dimer. Upon addition of a PPIase cochaperone to the Hsp90–Sti1 complex, an asymmetric ternary complex is preferentially formed. This PPIase–Hsp90–Sti1 intermediate is important for the progression of the cycle. To expel the bound Sti1, the concerted action of ATP and p23 is required. This mechanism, which is strictly conserved between the yeast and human Hsp90 systems, presents an example of how, in a cyclic process, directionality of assembly and disassembly of protein complexes can be achieved.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Borkovich, K.A., Farrelly, F.W., Finkelstein, D.B., Taulien, J. & Lindquist, S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol. Cell. Biol. 9, 3919–3930 (1989).
Dezwaan, D.C. & Freeman, B.C. HSP90: the Rosetta stone for cellular protein dynamics? Cell Cycle 7, 1006–1012 (2008).
Taipale, M., Jarosz, D.F. & Lindquist, S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 11, 515–528 (2010).
Young, J.C., Moarefi, I. & Hartl, F.U. Hsp90: a specialized but essential protein-folding tool. J. Cell Biol. 154, 267–273 (2001).
Csermely, P., Schnaider, T., Soti, C., Prohaszka, Z. & Nardai, G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol. Ther. 79, 129–168 (1998).
Welch, W.J. & Feramisco, J.R. Purification of the major mammalian heat shock proteins. J. Biol. Chem. 257, 14949–14959 (1982).
Geller, R., Vignuzzi, M., Andino, R. & Frydman, J. Evolutionary constraints on chaperone-mediated folding provide an antiviral approach refractory to development of drug resistance. Genes Dev. 21, 195–205 (2007).
Mayer, M.P. & Bukau, B. Molecular chaperones: the busy life of Hsp90. Curr. Biol. 9, R322–R325 (1999).
McClellan, A.J. et al. Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell 131, 121–135 (2007).
Pratt, W.B. & Toft, D.O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. (Maywood) 228, 111–133 (2003).
Cunningham, C.N., Krukenberg, K.A. & Agard, D.A. Intra- and intermonomer interactions are required to synergistically facilitate ATP hydrolysis in Hsp90. J. Biol. Chem. 283, 21170–21178 (2008).
Graf, C., Stankiewicz, M., Kramer, G. & Mayer, M.P. Spatially and kinetically resolved changes in the conformational dynamics of the Hsp90 chaperone machine. EMBO J. 28, 602–613 (2009).
Phillips, J.J. et al. Conformational dynamics of the molecular chaperone Hsp90 in complexes with a co-chaperone and anticancer drugs. J. Mol. Biol. 372, 1189–1203 (2007).
Shiau, A.K., Harris, S.F., Southworth, D.R. & Agard, D.A. Structural analysis of E. coli hsp90 reveals dramatic nucleotide-dependent conformational rearrangements. Cell 127, 329–340 (2006).
Picard, D. Heat-shock protein 90, a chaperone for folding and regulation. Cell. Mol. Life Sci. 59, 1640–1648 (2002).
Pratt, W.B. & Toft, D.O. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 18, 306–360 (1997).
Wandinger, S.K., Richter, K. & Buchner, J. The Hsp90 chaperone machinery. J. Biol. Chem. 283, 18473–18477 (2008).
Cox, M.B. et al. FK506-binding protein 52 phosphorylation: a potential mechanism for regulating steroid hormone receptor activity. Mol. Endocrinol. 21, 2956–2967 (2007).
Johnson, J.L. & Toft, D.O. A novel chaperone complex for steroid receptors involving heat shock proteins, immunophilins, and p23. J. Biol. Chem. 269, 24989–24993 (1994).
Mayr, C., Richter, K., Lilie, H. & Buchner, J. Cpr6 and Cpr7, two closely related Hsp90-associated immunophilins from Saccharomyces cerevisiae, differ in their functional properties. J. Biol. Chem. 275, 34140–34146 (2000).
Pirkl, F. & Buchner, J. Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and Cyp40. J. Mol. Biol. 308, 795–806 (2001).
Riggs, D.L. et al. Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling. Mol. Cell. Biol. 27, 8658–8669 (2007).
Schiene, C. & Fischer, G. Enzymes that catalyse the restructuring of proteins. Curr. Opin. Struct. Biol. 10, 40–45 (2000).
D'Andrea, L.D. & Regan, L. TPR proteins: the versatile helix. Trends Biochem. Sci. 28, 655–662 (2003).
Scheufler, C. et al. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell 101, 199–210 (2000).
Chen, S., Sullivan, W.P., Toft, D.O. & Smith, D.F. Differential interactions of p23 and the TPR-containing proteins Hop, Cyp40, FKBP52 and FKBP51 with Hsp90 mutants. Cell Stress Chaperones 3, 118–129 (1998).
Picard, D. et al. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature 348, 166–168 (1990).
Smith, D.F., Stensgard, B.A., Welch, W.J. & Toft, D.O. Assembly of progesterone receptor with heat shock proteins and receptor activation are ATP mediated events. J. Biol. Chem. 267, 1350–1356 (1992).
Cheung-Flynn, J. et al. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol. Endocrinol. 19, 1654–1666 (2005).
Johnson, J.L., Beito, T.G., Krco, C.J. & Toft, D.O. Characterization of a novel 23-kilodalton protein of unactive progesterone receptor complexes. Mol. Cell. Biol. 14, 1956–1963 (1994).
Smith, D.F. Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Mol. Endocrinol. 7, 1418–1429 (1993).
Chen, S. & Smith, D.F. Hop as an adaptor in the heat shock protein 70 (Hsp70) and hsp90 chaperone machinery. J. Biol. Chem. 273, 35194–35200 (1998).
Forafonov, F. et al. p23/Sba1p protects against Hsp90 inhibitors independently of its intrinsic chaperone activity. Mol. Cell. Biol. 28, 3446–3456 (2008).
Freeman, B.C., Felts, S.J., Toft, D.O. & Yamamoto, K.R. The p23 molecular chaperones act at a late step in intracellular receptor action to differentially affect ligand efficacies. Genes Dev. 14, 422–434 (2000).
Johnson, J.L. & Toft, D.O. Binding of p23 and hsp90 during assembly with the progesterone receptor. Mol. Endocrinol. 9, 670–678 (1995).
McLaughlin, S.H. et al. The co-chaperone p23 arrests the Hsp90 ATPase cycle to trap client proteins. J. Mol. Biol. 356, 746–758 (2006).
Prodromou, C. et al. Regulation of Hsp90 ATPase activity by tetratricopeptide repeat (TPR)-domain co-chaperones. EMBO J. 18, 754–762 (1999).
Richter, K., Muschler, P., Hainzl, O., Reinstein, J. & Buchner, J. Sti1 is a non-competitive inhibitor of the Hsp90 ATPase. Binding prevents the N-terminal dimerization reaction during the atpase cycle. J. Biol. Chem. 278, 10328–10333 (2003).
Ali, M.M. et al. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature 440, 1013–1017 (2006).
Johnson, J.L., Halas, A. & Flom, G. Nucleotide-dependent interaction of Saccharomyces cerevisiae Hsp90 with the cochaperone proteins Sti1, Cpr6, and Sba1. Mol. Cell. Biol. 27, 768–776 (2007).
Hessling, M., Richter, K. & Buchner, J. Dissection of the ATP-induced conformational cycle of the molecular chaperone Hsp90. Nat. Struct. Mol. Biol. 16, 287–293 (2009).
Yi, F., Doudevski, I. & Regan, L. HOP is a monomer: investigation of the oligomeric state of the co-chaperone HOP. Protein Sci. 19, 19–25 (2010).
Kroe, R.R. & Laue, T.M. NUTS and BOLTS: applications of fluorescence-detected sedimentation. Anal. Biochem. 390, 1–13 (2009).
Demeler, B. Methods for the design and analysis of sedimentation velocity and sedimentation equilibrium experiments with proteins. Curr. Protoc. Protein Sci. 60, 7.13.1–7.13.24 (2010).
Flom, G., Weekes, J., Williams, J.J. & Johnson, J.L. Effect of mutation of the tetratricopeptide repeat and asparatate-proline 2 domains of Sti1 on Hsp90 signaling and interaction in Saccharomyces cerevisiae. Genetics 172, 41–51 (2006).
Chadli, A. et al. Dimerization and N-terminal domain proximity underlie the function of the molecular chaperone heat shock protein 90. Proc. Natl. Acad. Sci. USA 97, 12524–12529 (2000).
Grenert, J.P., Johnson, B.D. & Toft, D.O. The importance of ATP binding and hydrolysis by hsp90 in formation and function of protein heterocomplexes. J. Biol. Chem. 274, 17525–17533 (1999).
Koulov, A.V. et al. Biological and structural basis for Aha1 regulation of Hsp90 ATPase activity in maintaining proteostasis in the human disease cystic fibrosis. Mol. Biol. Cell 21, 871–884 (2010).
Retzlaff, M. et al. Asymmetric activation of the hsp90 dimer by its cochaperone aha1. Mol. Cell 37, 344–354 (2010).
Ghaemmaghami, S. et al. Global analysis of protein expression in yeast. Nature 425, 737–741 (2003).
Chang, H.C., Nathan, D.F. & Lindquist, S. In vivo analysis of the Hsp90 cochaperone Sti1 (p60). Mol. Cell. Biol. 17, 318–325 (1997).
Owens-Grillo, J.K. et al. A model of protein targeting mediated by immunophilins and other proteins that bind to hsp90 via tetratricopeptide repeat domains. J. Biol. Chem. 271, 13468–13475 (1996).
Eisen, M.B., Spellman, P.T., Brown, P.O. & Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95, 14863–14868 (1998).
Gasch, A.P. et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11, 4241–4257 (2000).
Jakob, U. et al. Structural organization of procaryotic and eucaryotic Hsp90. Influence of divalent cations on structure and function. J. Biol. Chem. 270, 14412–14419 (1995).
Richter, K., Walter, S. & Buchner, J. The co-chaperone Sba1 connects the ATPase reaction of Hsp90 to the progression of the chaperone cycle. J. Mol. Biol. 342, 1403–1413 (2004).
Ali, J.A., Jackson, A.P., Howells, A.J. & Maxwell, A. The 43-kilodalton N-terminal fragment of the DNA gyrase B protein hydrolyzes ATP and binds coumarin drugs. Biochemistry 32, 2717–2724 (1993).
Richter, K., Muschler, P., Hainzl, O. & Buchner, J. Coordinated ATP hydrolysis by the Hsp90 dimer. J. Biol. Chem. 276, 33689–33696 (2001).
Winzeler, E.A. et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285, 901–906 (1999).
Stafford, W.F. III. Boundary analysis in sedimentation transport experiments: a procedure for obtaining sedimentation coefficient distributions using the time derivative of the concentration profile. Anal. Biochem. 203, 295–301 (1992).
Acknowledgements
We thank A. Kazlauskas (Karolinska Institute) for the AIP cDNA template, A. Schmid (Technische Universität München) for providing the Sti1 mutant R341E, and Y. Le and J. Winter (Technische Universität München) for the gift of YjiE. J.B. and K.R. were supported by the Deutsche Forschungsgemeinschaft (grants SFB594 A2 and RI1873/1-1, respectively) and by the Fonds der chemischen Industrie.
Author information
Authors and Affiliations
Contributions
J.L. designed, performed and analyzed experiments and wrote the first draft of the paper. K.R. was responsible for aUC and data analysis and contributed to writing the manuscript. J.B. designed and supervised experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Methods (PDF 1120 kb)
Rights and permissions
About this article
Cite this article
Li, J., Richter, K. & Buchner, J. Mixed Hsp90–cochaperone complexes are important for the progression of the reaction cycle. Nat Struct Mol Biol 18, 61–66 (2011). https://doi.org/10.1038/nsmb.1965
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1965
This article is cited by
-
Structural elements in the flexible tail of the co-chaperone p23 coordinate client binding and progression of the Hsp90 chaperone cycle
Nature Communications (2021)
-
The Hsp70–Hsp90 go-between Hop/Stip1/Sti1 is a proteostatic switch and may be a drug target in cancer and neurodegeneration
Cellular and Molecular Life Sciences (2021)
-
A methylated lysine is a switch point for conformational communication in the chaperone Hsp90
Nature Communications (2020)
-
Glucocorticoid receptor complexes form cooperatively with the Hsp90 co-chaperones Pp5 and FKBPs
Scientific Reports (2020)
-
The Hsp90 isoforms from S. cerevisiae differ in structure, function and client range
Nature Communications (2019)