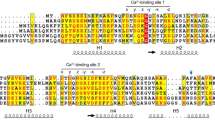
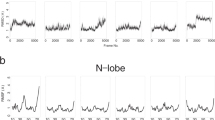
Abstract
Calcium-dependent protein kinases (CDPKs) have pivotal roles in the calcium-signaling pathway in plants, ciliates and apicomplexan parasites and comprise a calmodulin-dependent kinase (CaMK)-like kinase domain regulated by a calcium-binding domain in the C terminus. To understand this intramolecular mechanism of activation, we solved the structures of the autoinhibited (apo) and activated (calcium-bound) conformations of CDPKs from the apicomplexan parasites Toxoplasma gondii and Cryptosporidium parvum. In the apo form, the C-terminal CDPK activation domain (CAD) resembles a calmodulin protein with an unexpected long helix in the N terminus that inhibits the kinase domain in the same manner as CaMKII. Calcium binding triggers the reorganization of the CAD into a highly intricate fold, leading to its relocation around the base of the kinase domain to a site remote from the substrate binding site. This large conformational change constitutes a distinct mechanism in calcium signal-transduction pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sibley, L.D. Intracellular parasite invasion strategies. Science 304, 248–253 (2004).
Nagamune, K., Moreno, S.N., Chini, E.N. & Sibley, L.D. Calcium regulation and signaling in apicomplexan parasites. Subcell. Biochem. 47, 70–81 (2008).
Rosenberg, O.S. et al. Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell 123, 849–860 (2005).
Billker, O., Lourido, S. & Sibley, L.D. Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe 5, 612–622 (2009).
Qiu, W. et al. Novel structural and regulatory features of rhoptry secretory kinases in Toxoplasma gondii. EMBO J. 28, 969–979 (2009).
Cowan-Jacob, S.W. et al. The crystal structure of a c-Src complex in an active conformation suggests possible steps in c-Src activation. Structure 13, 861–871 (2005).
Nagar, B. et al. Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell 112, 859–871 (2003).
Chandran, V. et al. Structure of the regulatory apparatus of a calcium-dependent protein kinase (CDPK): a novel mode of calmodulin-target recognition. J. Mol. Biol. 357, 400–410 (2006).
Christodoulou, J., Malmendal, A., Harper, J.F. & Chazin, W.J. Evidence for differing roles for each lobe of the calmodulin-like domain in a calcium-dependent protein kinase. J. Biol. Chem. 279, 29092–29100 (2004).
Harmon, A.C., Putnam-Evans, C. & Cormier, M.J.A. Calcium-dependent but calmodulin-independent protein kinase from soybean. Plant Physiol. 83, 830–837 (1987).
Ranjan, R., Ahmed, A., Gourinath, S. & Sharma, P. Dissection of mechanisms involved in the regulation of Plasmodium falciparum calcium dependent protein kinase 4 (PfCDPK4). J. Biol. Chem. 284, 15267–15276 (2009).
Billker, O. et al. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell 117, 503–514 (2004).
Green, J.L. et al. The motor complex of Plasmodium falciparum: phosphorylation by a calcium-dependent protein kinase. J. Biol. Chem. 283, 30980–30989 (2008).
Dolle, C. & Ziegler, M. Application of a coupled enzyme assay to characterize nicotinamide riboside kinases. Anal. Biochem. 385, 377–379 (2009).
Harmon, A.C., Yoo, B.C. & McCaffery, C. Pseudosubstrate inhibition of CDPK, a protein kinase with a calmodulin-like domain. Biochemistry 33, 7278–7287 (1994).
Yoo, B.C. & Harmon, A.C. Intramolecular binding contributes to the activation of CDPK, a protein kinase with a calmodulin-like domain. Biochemistry 35, 12029–12037 (1996).
Meador, W.E., Means, A.R. & Quiocho, F.A. Modulation of calmodulin plasticity in molecular recognition on the basis of X-ray structures. Science 262, 1718–1721 (1993).
Chattopadhyaya, R., Meador, W.E., Means, A.R. & Quiocho, F.A. Calmodulin structure refined at 1.7 Å resolution. J. Mol. Biol. 228, 1177–1192 (1992).
Vitart, V. et al. Intramolecular activation of a Ca2+-dependent protein kinase is disrupted by insertions in the tether that connects the calmodulin-like domain to the kinase. Biochemistry 39, 4004–4011 (2000).
Hegeman, A.D. et al. A phyloproteomic characterization of in vitro autophosphorylation in calcium-dependent protein kinases. Proteomics 6, 3649–3664 (2006).
Knight, Z.A. & Shokat, K.M. Features of selective kinase inhibitors. Chem. Biol. 12, 621–637 (2005).
Ojo, K.K. et al. A unique variation of the ATP binding site makes Toxoplasma gondii calcium-dependent protein kinase 1 a drug target for selective kinase inhibitors. Nat. Struct. Mol. Biol. advance online publication, doi:10.1038/nsmb.1818 (2 May 2010).
Braun, A.P. & Schulman, H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu. Rev. Physiol. 57, 417–445 (1995).
Hanson, P.I., Meyer, T., Stryer, L. & Schulman, H. Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron 12, 943–956 (1994).
Parsons, M., Worthey, E.A., Ward, P.N. & Mottram, J.C. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genomics 6, 127 (2005).
Vedadi, M. et al. Genome-scale protein expression and structural biology of Plasmodium falciparum and related apicomplexan organisms. Mol. Biochem. Parasitol. 151, 100–110 (2007).
Terwilliger, T. SOLVE and RESOLVE: automated structure solution, density modification and model building. J. Synchrotron Radiat. 11, 49–52 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Kiianitsa, K., Solinger, J.A. & Heyer, W.D. NADH-coupled microplate photometric assay for kinetic studies of ATP-hydrolyzing enzymes with low and high specific activities. Anal. Biochem. 321, 266–271 (2003).
Acknowledgements
We thank J. Christodoulou, O. Billker, S. Knapp, T. Heightman, M. Schapira, C. Arrowsmith and A. Edwards for insightful discussions and/or constructive review of this manuscript and J. Lew, A. Hutchinson, A. Hassanali and H. Ren for their efforts in cloning and expressing the proteins in this study. The Structural Genomics Consortium is a registered charity (number 1097737) that receives funds from the Canadian Institutes for Health Research, the Canadian Foundation for Innovation, Genome Canada through the Ontario Genomics Institute, GlaxoSmithKline, the Knut and Alice Wallenberg Foundation, the Ontario Innovation Trust, the Ontario Ministry for Research and Innovation, Merck & Co. Inc., the Novartis Research Foundation, the Petrus and Augusta Hedlund's Foundation, the Swedish Agency for Innovation Systems, the Swedish Foundation for Strategic Research and the Wellcome Trust. This work was also partially supported by a US National Institutes of Health grant (AI034036) to L.D.S.
Author information
Authors and Affiliations
Contributions
Y.L. and M.A. purified and crystallized the proteins and performed MS analyses; A.K.W. and W.T. collected and processed X-ray data; A.K.W., J.D.A. and R.H. designed the experiments and analyzed the results; A.K.W. refined and analyzed the structural models; F.M. cloned the constructs; S.L. and L.D.S. identified the entry clone for TgCDPK1; L.D.S. provided functional analysis; P.F. Jr., A.A.-H., G.S. and I.C. conducted various assays on the proteins and analyzed the results; M.V. was involved in analysis of assay results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 1654 kb)
Rights and permissions
About this article
Cite this article
Wernimont, A., Artz, J., Finerty, P. et al. Structures of apicomplexan calcium-dependent protein kinases reveal mechanism of activation by calcium. Nat Struct Mol Biol 17, 596–601 (2010). https://doi.org/10.1038/nsmb.1795
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1795
This article is cited by
-
An update on Cryptosporidium biology and therapeutic avenues
Journal of Parasitic Diseases (2022)
-
Proteins with calmodulin-like domains: structures and functional roles
Cellular and Molecular Life Sciences (2019)
-
Protein targets of thiazolidinone derivatives in Toxoplasma gondii and insights into their binding to ROP18
BMC Genomics (2018)
-
The calcium-dependent protein kinase gene VaCPK29 is involved in grapevine responses to heat and osmotic stresses
Plant Growth Regulation (2017)
-
WDRP, a DWD protein component of CUL4-based E3 ligases, acts as a receptor of CDPK-related protein kinase 5 to mediate kinase degradation in Arabidopsis
Journal of Plant Biology (2016)