Abstract
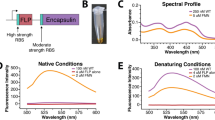
Compartmentalization is an important organizational feature of life. It occurs at varying levels of complexity ranging from eukaryotic organelles and the bacterial microcompartments, to the molecular reaction chambers formed by enzyme assemblies. The structural basis of enzyme encapsulation in molecular compartments is poorly understood. Here we show, using X-ray crystallographic, biochemical and EM experiments, that a widespread family of conserved bacterial proteins, the linocin-like proteins, form large assemblies that function as a minimal compartment to package enzymes. We refer to this shell-forming protein as 'encapsulin'. The crystal structure of such a particle from Thermotoga maritima determined at 3.1-Å resolution reveals that 60 copies of the monomer assemble into a thin, icosahedral shell with a diameter of 240 Å. The interior of this nanocompartment is lined with conserved binding sites for short polypeptide tags present as C-terminal extensions of enzymes involved in oxidative-stress response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cannon, G.C. et al. Microcompartments in prokaryotes: carboxysomes and related polyhedra. Appl. Environ. Microbiol. 67, 5351–5361 (2001).
Kerfeld, C.A. et al. Protein structures forming the shell of primitive bacterial organelles. Science 309, 936–938 (2005).
Tanaka, S. et al. Atomic-level models of the bacterial carboxysome shell. Science 319, 1083–1086 (2008).
Havemann, G.D., Sampson, E.M. & Bobik, T.A. PduA is a shell protein of polyhedral organelles involved in coenzyme B12-dependent degradation of 1,2-propanediol in Salmonella enterica serovar typhimurium LT2. J. Bacteriol. 184, 1253–1261 (2002).
Kofoid, E., Rappleye, C., Stojiljkovic, I. & Roth, J. The 17-gene ethanolamine (eut) operon of Salmonella typhimurium encodes five homologues of carboxysome shell proteins. J. Bacteriol. 181, 5317–5329 (1999).
Anderson, D.H., Kickhoefer, V.A., Sievers, S.A., Rome, L.H. & Eisenberg, D. Draft crystal structure of the vault shell at 9- resolution. PLoS Biol. 5, e318 (2007).
Smith, J.L. The physiological role of ferritin-like compounds in bacteria. Crit. Rev. Microbiol. 30, 173–185 (2004).
Ævarsson, A., Seger, K., Turley, S., Sokatch, J.R. & Hol, W.G. Crystal structure of 2-oxoisovalerate and dehydrogenase and the architecture of 2-oxo acid dehydrogenase multienzyme complexes. Nat. Struct. Biol. 6, 785–792 (1999).
Ritsert, K. et al. Studies on the lumazine synthase/riboflavin synthase complex of Bacillus subtilis: crystal structure analysis of reconstituted, icosahedral β-subunit capsids with bound substrate analogue inhibitor at 2.4 resolution. J. Mol. Biol. 253, 151–167 (1995).
Jenni, S. et al. Structure of fungal fatty acid synthase and implications for iterative substrate shuttling. Science 316, 254–261 (2007).
Valdes-Stauber, N. & Scherer, S. Isolation and characterization of Linocin M18, a bacteriocin produced by Brevibacterium linens. Appl. Environ. Microbiol. 60, 3809–3814 (1994).
Hicks, P.M., Rinker, K.D., Baker, J.R. & Kelly, R.M. Homomultimeric protease in the hyperthermophilic bacterium Thermotoga maritima has structural and amino acid sequence homology to bacteriocins in mesophilic bacteria. FEBS Lett. 440, 393–398 (1998).
Rosenkrands, I. et al. Identification and characterization of a 29-kilodalton protein from Mycobacterium tuberculosis culture filtrate recognized by mouse memory effector cells. Infect. Immun. 66, 2728–2735 (1998).
Wikoff, W.R. et al. Topologically linked protein rings in the bacteriophage HK97 capsid. Science 289, 2129–2133 (2000).
Akita, F. et al. The crystal structure of a virus-like particle from the hyperthermophilic archaeon Pyrococcus furiosus provides insight into the evolution of viruses. J. Mol. Biol. 368, 1469–1483 (2007).
Helgstrand, C. et al. The refined structure of a protein catenane: the HK97 bacteriophage capsid at 3.44 resolution. J. Mol. Biol. 334, 885–899 (2003).
Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Altschul, S.F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Kim, S.J. & Shoda, M. Purification and characterization of a novel peroxidase from Geotrichum candidum Dec 1 involved in decolorization of dyes. Appl. Environ. Microbiol. 65, 1029–1035 (1999).
Chang, C., Evdokimova, E., Savchenko, A. & Joachimiak, A. & Midwest Center for Structural Genomics. Crystal structure of protein NE0167 from Nitrosomonas europaea, PDB 1ZPY, doi:102210/pdb1zpy/pdb (2005).
Marcotte, E.M. et al. Detecting protein function and protein-protein interactions from genome sequences. Science 285, 751–753 (1999).
Valdes-Stauber, N. & Scherer, S. Nucleotide sequence and taxonomical distribution of the bacteriocin gene lin cloned from Brevibacterium linens M18. Appl. Environ. Microbiol. 62, 1283–1286 (1996).
Zubieta, C. et al. Crystal structures of two novel dye-decolorizing peroxidases reveal a β-barrel fold with a conserved heme-binding motif. Proteins 69, 223–233 (2007).
Bamford, D.H., Grimes, J.M. & Stuart, D.I. What does structure tell us about virus evolution? Curr. Opin. Struct. Biol. 15, 655–663 (2005).
Takahashi, T. & Kuyucak, S. Functional properties of threefold and fourfold channels in ferritin deduced from electrostatic calculations. Biophys. J. 84, 2256–2263 (2003).
Harrison, P.M. & Arosio, P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1275, 161–203 (1996).
Rocha, E.R., Andrews, S.C., Keen, J.N. & Brock, J.H. Isolation of a ferritin from Bacteroides fragilis. FEMS Microbiol. Lett. 95, 207–212 (1992).
Finegold, S.M. & George, W.L. Anaerobic Infections in Humans Vol. XXI 851 (Academic Press, San Diego, CA, 1989).
Manca, C., Paul, S., Barry, C.E. III, Freedman, V.H. & Kaplan, G. Mycobacterium tuberculosis catalase and peroxidase activities and resistance to oxidative killing in human monocytes in vitro. Infect. Immun. 67, 74–79 (1999).
Tsai, Y. et al. Structural analysis of CsoS1A and the protein shell of the Halothiobacillus neapolitanus carboxysome. PLoS Biol. 5, e144 (2007).
Yeates, T.O., Tsai, Y., Tanaka, S., Sawaya, M.R. & Kerfeld, C.A. Self-assembly in the carboxysome: a viral capsid-like protein shell in bacterial cells. Biochem. Soc. Trans. 35, 508–511 (2007).
Tobimatsu, T., Kawata, M. & Toraya, T. The N-terminal regions of β and γ subunits lower the solubility of adenosylcobalamin-dependent diol dehydratase. Biosci. Biotechnol. Biochem. 69, 455–462 (2005).
Stauber, N.V. Isolierung, Charakterisierung und Nukleinsäuresequenz eines Bakteriozins aus Brevibacterium linens M18. Doktor der Naturwissenschaften Thesis, Technische Universität München-Weihenstephan, (1995).
Seebeck, F.P., Woycechowsky, K.J., Zhuang, W., Rabe, J.P. & Hilvert, D. A simple tagging system for protein encapsulation. J. Am. Chem. Soc. 128, 4516–4517 (2006).
Huber, R. et al. Thermotoga maritima sp. nov represents a new genus of unique extremely thermophilic eubacteria growing up to 90 °C. Arch. Microbiol. 144, 324–333 (1986).
Santimone, M. Titration study of guaiacol oxidation by horseradish peroxidase. Can. J. Biochem. 53, 649–657 (1975).
Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 26, 795–800 (1993).
Rossmann, M.G. & Blow, D.M. The detection of sub-units within the crystallographic asymmetric unit. Acta Crystallogr. 15, 24–31 (1962).
Tong, L. & Rossmann, M.G. Rotation function calculations with GLRF program. Methods Enzymol. 276, 594–611 (1997).
Kleywegt, G.J. & Read, R.J. Not your average density. Structure 5, 1557–1569 (1997).
Cowtan, K.D. & Main, P. Improvement of macromolecular electron-density maps by the simultaneous application of real and reciprocal space constraints. Acta Crystallogr. D Biol. Crystallogr. 49, 148–157 (1993).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Afonine, P.V., Grosse-Kunstleve, R.W. & Adams, P.D. CCP4 Newsletter No. 42, Contribution 8, (Daresbury Laboratory, Warrington, Cheshire, UK, 2005).
Krissinel, E. & Henrick, K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 60, 2256–2268 (2004).
Ohi, M., Li, Y., Cheng, Y. & Walz, T. Negative staining and image classification—powerful tools in modern electron microscopy. Biol. Proced. Online 6, 23–34 (2004).
Nickell, S. et al. TOM software toolbox: acquisition and analysis for electron tomography. J. Struct. Biol. 149, 227–234 (2005).
Sander, B., Golas, M.M. & Stark, H. Automatic CTF correction for single particles based upon multivariate statistical analysis of individual power spectra. J. Struct. Biol. 142, 392–401 (2003).
van Heel, M., Harauz, G., Orlova, E.V., Schmidt, R. & Schatz, M. A new generation of the IMAGIC image processing system. J. Struct. Biol. 116, 17–24 (1996).
Dube, P., Tavares, P., Lurz, R. & van Heel, M. The portal protein of bacteriophage SPP1: a DNA pump with 13-fold symmetry. EMBO J. 12, 1303–1309 (1993).
van Heel, M. & Frank, J. Use of multivariate statistics in analysing the images of biological macromolecules. Ultramicroscopy 6, 187–194 (1981).
van Heel, M. Angular reconstitution: a posteriori assignment of projection directions for 3D reconstruction. Ultramicroscopy 21, 111–123 (1987).
Orlova, E.V. et al. Structure of keyhole limpet hemocyanin type 1 (KLH1) at 15 resolution by electron cryomicroscopy and angular reconstitution. J. Mol. Biol. 271, 417–437 (1997).
Sali, A. & Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Wriggers, W., Milligan, R.A. & McCammon, J.A. Situs: a package for docking crystal structures into low-resolution maps from electron microscopy. J. Struct. Biol. 125, 185–195 (1999).
Barton, G.J. ALSCRIPT: a tool to format multiple sequence alignments. Protein Eng. 6, 37–40 (1993).
Acknowledgements
Crystallographic data were collected at the beamline X06SA at the Swiss Light Source (SLS). We are grateful to C. Schulze-Briese, E. Pohl and T. Tomizaki for their outstanding support at the SLS. We thank R. Brunisholz, P. Hunziker and Y. Auchli at the Functional Genomics Center Zurich for mass-spectrometric analysis, the Electron Microscopy Center Zurich (EMEZ) and Martin Beck for support with EM data collection, M. Müller, F. Voigts-Hoffmann and F. Imkamp for critically reading the manuscript and all members of the Ban and Weber-Ban laboratory for suggestions and discussions. D.B. was supported by a Federation of European Biochemical Societies long-term fellowship. This work was supported by the Swiss National Science Foundation (SNSF) (to N.B. and E.W.B.) and the National Center of Excellence in Research (NCCR) Structural Biology program of the SNSF (to N.B. and E.W.B.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors are planning on filing a pre-patent application on possible uses and applications of encapsulin in biotechnology and biomedicine.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and Supplementary data (PDF 1461 kb)
Rights and permissions
About this article
Cite this article
Sutter, M., Boehringer, D., Gutmann, S. et al. Structural basis of enzyme encapsulation into a bacterial nanocompartment. Nat Struct Mol Biol 15, 939–947 (2008). https://doi.org/10.1038/nsmb.1473
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1473
This article is cited by
-
Linocin M18 protein from the insect pathogenic bacterium Brevibacillus laterosporus isolates
Applied Microbiology and Biotechnology (2023)
-
Adaptation of a microbial community to demand-oriented biological methanation
Biotechnology for Biofuels and Bioproducts (2022)
-
Mutations in respiratory complex I promote antibiotic persistence through alterations in intracellular acidity and protein synthesis
Nature Communications (2022)
-
Exploring targeting peptide-shell interactions in encapsulin nanocompartments
Scientific Reports (2021)
-
Large-scale computational discovery and analysis of virus-derived microbial nanocompartments
Nature Communications (2021)