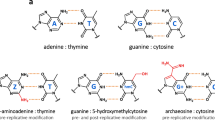
Abstract
Chlorella virus DNA ligase, the smallest eukaryotic ligase known, has pluripotent biological activity and an intrinsic nick-sensing function, despite having none of the accessory domains found in cellular ligases. A 2.3-Å crystal structure of the Chlorella virus ligase-AMP intermediate bound to duplex DNA containing a 3′-OH–5′-PO4 nick reveals a new mode of DNA envelopment, in which a short surface loop emanating from the OB domain forms a β-hairpin 'latch' that inserts into the DNA major groove flanking the nick. A network of interactions with the 3′-OH and 5′-PO4 termini in the active site illuminates the DNA adenylylation mechanism and the crucial roles of AMP in nick sensing and catalysis. Addition of a divalent cation triggered nick sealing in crystallo, establishing that the nick complex is a bona fide intermediate in the DNA repair pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gellert, M. Formation of covalent circles of lambda DNA by E. coli extracts. Proc. Natl. Acad. Sci. USA 57, 148–155 (1967).
Zimmerman, S.B., Little, J.W., Oshinsky, C.K. & Gellert, M. Enzymatic joining of DNA strands: a novel reaction of diphosphopyridine nucleotide. Proc. Natl. Acad. Sci. USA 57, 1841–1848 (1967).
Little, J.W., Zimmerman, S.B., Oshinsky, C.K. & Gellert, M. Enzymatic joining of DNA strands, II: an enzyme-adenylate intermediate in the DPN-dependent DNA ligase reaction. Proc. Natl. Acad. Sci. USA 58, 2004–2011 (1967).
Olivera, B.M. & Lehman, I.R. Linkage of polynucleotides through phosphodiester bonds by an enzyme from Escherichia coli. Proc. Natl. Acad. Sci. USA 57, 1426–1433 (1967).
Olivera, B.M. & Lehman, I.R. Diphosphopyridine nucleotide: a cofactor for the polynucleotide-joining enzyme from Escherichia coli. Proc. Natl. Acad. Sci. USA 57, 1700–1704 (1967).
Olivera, B.M., Hall, Z.W. & Lehman, I.R. Enzymatic joining of polynucleotides, V: a DNA-adenylate intermediate in the polynucleotide-joining reaction. Proc. Natl. Acad. Sci. USA 61, 237–244 (1968).
Weiss, B. & Richardson, C.C. Enzymatic breakage and joining of deoxyribonucleic acid, I: repair of single-strand breaks in DNA by and enzyme system from Escherichia coli infected with T4 bacteriophage. Proc. Natl. Acad. Sci. USA 57, 1021–1028 (1967).
Weiss, B. & Richardson, C.C. Enzymatic breakage and joining of deoxyribonucleic acid, III: an enzyme-adenylate intermediate in the polynucleotide ligase reaction. J. Biol. Chem. 242, 4270–4278 (1967).
Gefter, M.L., Becker, A. & Hurwitz, J. The enzymatic repair of DNA, I: formation of circular DNA. Proc. Natl. Acad. Sci. USA 58, 240–247 (1967).
Becker, A., Lyn, G., Gefter, M. & Hurwitz, J. The enzymatic repair of DNA, II: characterization of phaged-induced sealase. Proc. Natl. Acad. Sci. USA 58, 1996–2003 (1967).
Tomkinson, A.E., Vijayakumar, S., Pascal, J.M. & Ellenberger, T. DNA ligases: structure, reaction mechanism, and function. Chem. Rev. 106, 687–699 (2006).
Subramanya, H.S., Doherty, A.J., Ashford, S.R. & Wigley, D.B. Crystal structure of an ATP-dependent DNA ligase from bacteriophage T7. Cell 85, 607–615 (1996).
Lee, J.Y. et al. Crystal structure of NAD+-dependent DNA ligase: modular architecture and functional implications. EMBO J. 19, 1119–1129 (2000).
Odell, M., Sriskanda, V., Shuman, S. & Nikolov, D.B. Crystal structure of eukaryotic DNA ligase-adenylate illuminates the mechanism of nick sensing and strand joining. Mol. Cell 6, 1183–1193 (2000).
Pascal, J.M., O'Brien, P.J., Tomkinson, A.E. & Ellenberger, T. Human DNA ligase I completely encircles and partially unwinds nicked DNA. Nature 432, 473–478 (2004).
Akey, D. et al. Crystal structure and nonhomologous end joining function of the ligase domain of Mycobacterium DNA ligase D. J. Biol. Chem. 281, 13412–13423 (2006).
Pascal, J.M. et al. A flexible interface between DNA ligase and PCNA supports conformational switching and efficient ligation of DNA. Mol. Cell 24, 279–291 (2006).
Nishida, H., Kiyonari, S., Ishino, Y. & Morikawa, K. The closed structure of an archaeal DNA ligase from Pyrococcus furiosus. J. Mol. Biol. 360, 956–967 (2006).
Nandakumar, J., Nair, P.A. & Shuman, S. Last stop on the road to repair: structure of E. coli DNA ligase bound to nicked DNA-adenylate. Mol. Cell 26, 257–271 (2007).
Nandakumar, J., Shuman, S. & Lima, C.D. RNA ligase structures reveal the basis for RNA specificity and conformational changes that drive ligation forward. Cell 127, 71–84 (2006).
El Omari, K., Ren, J., Bird, L.E., Bona, M.K., Klarman, G., Legrice, S.F. & Stammers, D.K. Molecular architecture and ligand recognition determinants for T4 RNA ligase. J. Biol. Chem. 281, 1573–1579 (2006).
Håkansson, K., Doherty, A.J., Shuman, S. & Wigley, D.B. X-ray crystallography reveals a large conformational change during guanyl transfer by mRNA capping enzymes. Cell 89, 545–553 (1997).
Shuman, S. & Lima, C.D. The polynucleotide ligase and RNA capping enzyme superfamily of covalent nucleotidyltransferases. Curr. Opin. Struct. Biol. 14, 757–764 (2004).
Sriskanda, V. & Shuman, S. Mutational analysis of Chlorella virus DNA ligase: catalytic roles of domain I and motif VI. Nucleic Acids Res. 26, 4618–4625 (1998).
Sriskanda, V. & Shuman, S. Conserved residues in domain Ia are required for the reaction of Escherichia coli DNA ligase with NAD+. J. Biol. Chem. 277, 9695–9700 (2002).
Gajiwala, K.S. & Pinko, C. Structural rearrangement accompanying NAD+ synthesis within a bacterial DNA ligase crystal. Structure 12, 1449–1459 (2004).
Ho, C.K., Van Etten, J.L. & Shuman, S. Characterization of an ATP-dependent DNA ligase encoded by Chlorella virus PBCV-1. J. Virol. 71, 1931–1937 (1997).
Sriskanda, V. & Shuman, S. Chlorella virus DNA ligase: nick recognition and mutational analysis. Nucleic Acids Res. 26, 525–531 (1998).
Odell, M. & Shuman, S. Footprinting of Chlorella virus DNA ligase bound at a nick in duplex DNA. J. Biol. Chem. 274, 14032–14039 (1999).
Sekiguchi, J. & Shuman, S. Nick sensing by DNA ligase requires a 5′ phosphate at the nick and occupancy of the adenylate binding site on the enzyme. J. Virol. 71, 9679–9684 (1997).
Nandakumar, J. & Shuman, S. How an RNA ligase discriminates RNA versus DNA damage. Mol. Cell 16, 211–221 (2004).
Sriskanda, V., Schwer, B., Ho, C.K. & Shuman, S. Mutational analysis of E. coli DNA ligase identifies amino acids required for nick-ligation in vitro and for in vivo complementation of the growth of yeast cells deleted for CDC9 and LIG4. Nucleic Acids Res. 27, 3953–3963 (1999).
Odell, M., Malinina, L., Sriskanda, V., Teplova, M. & Shuman, S. Analysis of the DNA joining repertoire of Chlorella virus DNA ligase and a new crystal structure of the ligase-adenylate intermediate. Nucleic Acids Res. 31, 5090–5100 (2003).
Pascal, J.M. & Ellenberger, T. RNA ligase does the AMP shuffle. Nat. Struct. Mol. Biol. 13, 950–951 (2006).
Marchetti, C. et al. Identification of a novel motif in DNA ligases exemplified by DNA ligase IV. DNA Repair (Amst.) 5, 788–798 (2006).
Sriskanda, V. & Shuman, S. Specificity and fidelity of strand joining by Chlorella virus DNA ligase. Nucleic Acids Res. 26, 3536–3541 (1998).
Sriskanda, V. & Shuman, S. Role of nucleotidyltransferase motifs I, III and IV in the catalysis of phosphodiester bond formation by Chlorella virus DNA ligase. Nucleic Acids Res. 30, 903–911 (2002).
Nandakumar, J. & Shuman, S. Dual mechanisms whereby a broken RNA end assists the catalysis of its repair by T4 RNA ligase 2. J. Biol. Chem. 280, 23484–23489 (2005).
Shuman, S. Vaccinia DNA ligase: specificity, fidelity, and inhibition. Biochemistry 34, 16138–16147 (1995).
Ahel, I. et al. The neurodegenerative disease protein resolves abortive DNA ligation intermediates. Nature 443, 713–716 (2006).
Gong, C. et al. Mechanism of nonhomologous end-joining in mycobacteria: a low fidelity repair system driven by Ku, ligase D and ligase C. Nat. Struct. Mol. Biol. 12, 304–312 (2005).
Gong, C., Martins, A., Bongiorno, P., Glickman, M. & Shuman, S. Biochemical and genetic analysis of the four DNA ligases of mycobacteria. J. Biol. Chem. 279, 20594–20606 (2004).
Weller, G.R. et al. Identification of a DNA nonhomologous end-joining complex in bacteria. Science 297, 1686–1689 (2002).
Doherty, A.J., Ashford, S.R. & Wigley, D.B. Characterization of proteolytic fragments of bacteriophage T7 DNA ligase. Nucleic Acids Res. 24, 2281–2287 (1996).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Collaborative Computational Project. Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Brunger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the Maximum-Likelihood Method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–118 (1991).
Navaza, J. AMoRe: an automated package for molecular replacement. Acta Crystallogr. A 50, 157–163 (1994).
DeLano, W.L. The PyMOL Molecular Graphics System (DeLano Scientific, San Carlos, California, USA, 2002).
Acknowledgements
This paper is inspired by the 40th anniversary of the discovery of viral ATP-dependent DNA ligases by Charles Richardson and Jerry Hurwitz. This work was supported by US National Institutes of Health grants GM63611 (S.S.) and GM61906 (C.D.L.). S.S. is an American Cancer Society Research Professor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 2133 kb)
Rights and permissions
About this article
Cite this article
Nair, P., Nandakumar, J., Smith, P. et al. Structural basis for nick recognition by a minimal pluripotent DNA ligase. Nat Struct Mol Biol 14, 770–778 (2007). https://doi.org/10.1038/nsmb1266
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1266
This article is cited by
-
Structures of LIG1 that engage with mutagenic mismatches inserted by polβ in base excision repair
Nature Communications (2022)
-
Bacteriophage origin of some minimal ATP-dependent DNA ligases: a new structure from Burkholderia pseudomallei with striking similarity to Chlorella virus ligase
Scientific Reports (2021)
-
Structure of the error-prone DNA ligase of African swine fever virus identifies critical active site residues
Nature Communications (2019)
-
Temperature adaptation of DNA ligases from psychrophilic organisms
Extremophiles (2019)
-
Sequence-specific 1HN, 13C, and 15N backbone resonance assignments of the 34 kDa Paramecium bursaria Chlorella virus 1 (PBCV1) DNA ligase
Biomolecular NMR Assignments (2009)