Abstract
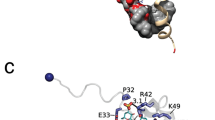
Thrombospondins (THBSs) are secreted glycoproteins that have key roles in interactions between cells and the extracellular matrix. Here, we describe the 2.6-Å-resolution crystal structure of the glycosylated signature domain of human THBS2, which includes three epidermal growth factor–like modules, 13 aspartate-rich repeats and a lectin-like module. These elements interact extensively to form three structural regions termed the stalk, wire and globe. The THBS2 signature domain is stabilized by these interactions and by a network of 30 bound Ca2+ ions and 18 disulfide bonds. The structure suggests how genetic alterations of THBSs result in disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Adams, J.C. & Lawler, J. The thrombospondins. Int. J. Biochem. Cell Biol. 36, 961–968 (2004).
Christopherson, K.S. et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433 (2005).
Kyriakides, T.R. et al. Megakaryocytes require thrombospondin-2 for normal platelet formation and function. Blood 101, 3915–3923 (2003).
Kyriakides, T.R., Zhu, Y.H., Yang, Z.T., Huynh, G. & Bornstein, P. Altered extracellular matrix remodeling and angiogenesis in sponge granulomas of thrombospondin 2-null mice. Am. J. Pathol. 159, 1255–1262 (2001).
Adams, J.C. et al. Characterisation of Drosophila thrombospondin defines an early origin of pentameric thrombospondins. J. Mol. Biol. 328, 479–494 (2003).
LaBell, T.L. & Byers, P.H. Sequence and characterization of the complete human thrombospondin 2 cDNA: potential regulatory role for the 3′ untranslated region. Genomics 17, 225–229 (1993).
Topol, E.J. et al. Single nucleotide polymorphisms in multiple novel thrombospondin genes may be associated with familial premature myocardial infarction. Circulation 104, 2641–2644 (2001).
Kennedy, J. et al. Novel and recurrent mutations in the C-terminal domain of COMP cluster in two distinct regions and result in a spectrum of phenotypes within the pseudoachondroplasia–multiple epiphyseal dysplasia disease group. Hum. Mutat. 25, 593–594 (2005).
Posey, K.L., Hayes, E., Haynes, R. & Hecht, J.T. Role of TSP-5/COMP in pseudoachondroplasia. Int. J. Biochem. Cell Biol. 36, 1005–1012 (2004).
Kvansakul, M., Adams, J.C. & Hohenester, E. Structure of a thrombospondin C-terminal fragment reveals a novel calcium core in the type 3 repeats. EMBO J. 23, 1223–1233 (2004).
Wouters, M.A. et al. Evolution of distinct EGF domains with specific functions. Protein Sci. 14, 1091–1103 (2005).
Boswell, E.J., Kurniawan, N.D. & Downing, A.K. Calcium-binding EGF-like domains. in Handbook of Metalloproteins Vol. 3 (eds. Messerschmidt, A., Bode, W. & Cygler, M.) 553–570 (Wiley, Chichester, UK, 2004).
Misenheimer, T.M., Hannah, B.L., Annis, D.S. & Mosher, D.F. Interactions among the three structural motifs of the C-terminal region of human thrombospondin-2. Biochemistry 42, 5125–5132 (2003).
Misenheimer, T.M., Hahr, A.J., Harms, A.C., Annis, D.S. & Mosher, D.F. Disulfide connectivity of recombinant C-terminal region of human thrombospondin 2. J. Biol. Chem. 276, 45882–45887 (2001).
Lawler, J., Chao, F.C. & Cohen, C.M. Evidence for calcium-sensitive structure in platelet thrombospondin: Isolation and partial characterization of thrombospondin in the presence of calcium. J. Biol. Chem. 257, 12257–12265 (1982).
Lawler, J., Derick, L.H., Connolly, J.E., Chen, J.-H. & Chao, F.C. The structure of human platelet thrombospondin. J. Biol. Chem. 260, 3762–3772 (1985).
Chen, H., Aeschlimann, D., Nowlen, J. & Mosher, D. Expression and initial characterization of recombinant mouse thrombospondin 1 and thrombospondin 3. FEBS Lett. 387, 36–41 (1996).
Lawler, J. & Hynes, R.O. An integrin receptor on normal and thrombasthenic platelets that binds thrombospondin. Blood 74, 2022–2027 (1989).
Chen, H., Sottile, J., O'Rourke, K.M., Dixit, V.M. & Mosher, D.F. Properties of recombinant mouse thrombospondin 2 expressed in Spodoptera cells. J. Biol. Chem. 269, 32226–32232 (1994).
Gao, A.G. & Frazier, W.A. Identification of a receptor candidate for the carboxyl-terminal cell binding domain of thrombospondins. J. Biol. Chem. 269, 29650–29657 (1994).
Hannah, B.L., Misenheimer, T.M., Pranghofer, M.M. & Mosher, D.F. A polymorphism in thrombospondin-1 associated with familial premature coronary artery disease alters Ca2+ binding. J. Biol. Chem. 279, 51915–51922 (2004).
Dinser, R. et al. Pseudoachondroplasia is caused through both intra-and extracellular pathogenic pathways. J. Clin. Invest. 110, 505–513 (2002).
Adams, J.C., Tucker, R.P. & Lawler, J. Mechanistic and functional aspects of the interactions of thrombospondins with cell surfaces. in The Thrombospondin Gene Family 105–157 (R.G. Landes Company, Austin, Texas, USA, 1995).
Mosher, D.F., Huwiler, K.G., Misenheimer, T.M. & Annis, D.S. Expression of recombinant matrix components using baculoviruses. Methods Cell Biol. 69, 69–81 (2002).
McWhirter, S.M. et al. Crystallographic analysis of CD40 recognition and signaling by human TRAF2. Proc. Natl. Acad. Sci. USA 96, 8408–8413 (1999).
Bellizzi, J.J., Widom, J., Kemp, C.W. & Clardy, J. Producing selenomethionine-labeled proteins with a baculovirus expression vector system. Struct. Fold. Des. 7, R263–R267 (1999).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Terwilliger, T.C. & Berendzen, J. Automated MAD and MIR structure solution. Acta Crystallogr. D Biol. Crystallogr. 55, 849–861 (1999).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Acknowledgements
We thank E. Hohenester for communicating results before publication and the Advanced Photon Source beamline staff for assistance in data collection. This work was supported by US National Institutes of Health grant HL54462 to D.F.M. and a Shaw Foundation for Medical Research grant to J.L.K. C.B.C. and D.A.B. were supported by US National Institutes of Health training grants HL07899 and GM08293.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Stereo diagram of the human TSP-2 signature domain structure (PDF 3045 kb)
Supplementary Fig. 2
Sequence and secondary structure of the signature domain of human TSP-2 (PDF 687 kb)
Supplementary Fig. 3
Comparison of the crystal structures of the TSP-2 signature domain and the TSP-1 signature domain fragment (PDF 1918 kb)
Supplementary Table 1
TSP-2 coordination of 30 bound Ca(2+) ions (PDF 890 kb)
Supplementary Table 2
Disease-linked TSP-family mutations (PDF 1189 kb)
Rights and permissions
About this article
Cite this article
Carlson, C., Bernstein, D., Annis, D. et al. Structure of the calcium-rich signature domain of human thrombospondin-2. Nat Struct Mol Biol 12, 910–914 (2005). https://doi.org/10.1038/nsmb997
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb997
This article is cited by
-
Molecular signatures of tumor progression in myxoid liposarcoma identified by N-glycan mass spectrometry imaging
Laboratory Investigation (2020)
-
The calcium-binding type III repeats domain of thrombospondin-2 binds to fibroblast growth factor 2 (FGF2)
Angiogenesis (2019)
-
COMP and TSP-4 interact specifically with the novel GXKGHR motif only found in fibrillar collagens
Scientific Reports (2018)
-
Role of Matricellular Proteins in Disorders of the Central Nervous System
Neurochemical Research (2017)
-
The Neuroligins and Their Ligands: from Structure to Function at the Synapse
Journal of Molecular Neuroscience (2014)