Abstract
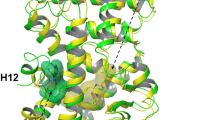
Retinoids regulate gene expression through binding to the nuclear retinoic acid receptors (RARs) and retinoid X receptors (RXRs). In contrast, no ligands for the retinoic acid receptor–related orphan receptors β and γ (RORβ and γ) have been identified, yet structural data and structure-function analyses indicate that RORβ is a ligand-regulated nuclear receptor. Using nondenaturing mass spectrometry and scintillation proximity assays we found that all-trans retinoic acid (ATRA) and several retinoids bind to the RORβ ligand-binding domain (LBD). The crystal structures of the complex with ATRA and with the synthetic analog ALRT 1550 reveal the binding modes of these ligands. ATRA and related retinoids inhibit RORβ but not RORα transcriptional activity suggesting that high-affinity, subtype-specific ligands could be designed for the identification of RORβ target genes. Our results identify RORβ as a retinoid-regulated nuclear receptor, providing a novel pathway for retinoid action.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jetten, A., Kurebayashi, S. & Ueda, E. The ROR nuclear orphan receptor subfamily: critical regulators of multiple biological processes. Prog. Nucl. Acid Res. Mol. Biol. 69, 205–247 (2001).
Preitner, N. et al. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 (2002).
Ueda, H.R. et al. A transcription factor response element for gene expression during circadian night. Nature 418, 534–539 (2002).
Greiner, E.F. et al. Functional analysis of retinoid Z receptor β, a brain-specific nuclear orphan receptor. Proc. Natl. Acad. Sci. USA 93, 10105–10110 (1996).
Greiner, E.F. et al. Differential ligand-dependent protein-protein interactions between nuclear receptors and a neuronal-specific cofactor. Proc. Natl. Acad. Sci. USA 97, 7160–7165 (2000).
Moraitis, A.N., Giguère, V. & Thompson, C.C. Novel mechanism of nuclear receptor corepressor interaction dictated by activation function 2 helix determinants. Mol. Cell. Biol. 22, 6831–6841 (2002).
Stehlin, C. et al. X-ray structure of the orphan nuclear receptor RORβ ligand-binding domain in the active conformation. EMBO J. 20, 5822–5831 (2001).
Potier, N. et al. Using non-denaturing mass spectrometry to detect fortuitous ligands in orphan receptors. Protein Sci. 12, 725–733 (2003).
Loo, J.A. Electrospray ionization mass spectrometry: a technology for studying noncovalent macromolecular complexes. Int. J. Mass Spectrom. 200, 175–186 (2000).
Renaud, J.P. et al. Crystal structure of the RAR-γ ligand-binding domain bound to all-trans retinoic acid. Nature 378, 681–689 (1995).
Kallen, J.A. et al. X-ray structure of the hRORα LBD at 1.63 Å. Structural and functional data that cholesterol or a cholesterol derivative is the natural ligand of RORα. Structure 10, 1697–1707 (2002).
Blumberg, B. et al. Novel retinoic acid receptor ligand in Xenopus embryos. Proc. Natl. Acad. Sci. USA 93, 4873–4878 (1996).
Kurlandsky, S.B. et al. Plasma delivery of retinoic acid to tissues in the rat. J. Biol. Chem. 270, 17850–17857 (1995).
Forman, B.M. et al. Androstane metabolites bind to and deactivate the nuclear receptor CAR-β. Nature 395, 612–615 (1998).
Tremblay, G.B. et al. Diethylstilbestrol regulates trophoblast stem cell differentiation as a ligand of orphan nuclear receptor ERRβ. Genes Dev. 15, 833–838 (2001).
Coward, P., Lee, D., Hull, M.V. & Lehmann, J. 4-Hydroxytamoxifen binds to and deactivates the estrogen-related receptor γ. Proc. Natl. Acad. Sci. USA 98, 8880–8884 (2001).
Greschik, H. et al. Structural and functional evidence for ligand-independent transcriptional activation by the estrogen-related receptor 3. Mol. Cell 9, 303–313 (2002).
Pike, A.C.W. et al. Structure of the ligand-binding domain of oestrogen receptor β in the presence of a partial agonist and a full antagonist. EMBO J. 18, 4608–4618 (1999).
Nolte, R.T. et al. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-γ. Nature 395, 137–143 (1998).
Watkins, R.E. et al. The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science 292, 2329–2333 (2001).
Bourguet, W. et al. Crystal structure of a heterodimeric complex of RAR and RXR ligand-binding domains. Mol. Cell 5, 289–298 (2000).
McNamara et al. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell 105, 877–889 (2001).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Navaza, J. AMoRe: an automated package for molecular replacement. Acta Crystallogr. A 50, 157–163 (1994).
Brünger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. A 47, 110–119 (1998).
Kleywegt, G.J. & Jones, T.A. Detection, delineation, measurement and display of cavities in macromolecular structures. Acta Crystallogr. D 50, 178–185 (1994).
Müller, J.M. et al. The transcriptional coactivator FHL2 transmits Rho signals from the cell membrane into the nucleus. EMBO J. 21, 736–748 (2002).
Geoghegan, K.F. et al. Spontaneous α-N-6-phosphogluconoylation of a 'His tag' in Escherichia coli: the cause of extra mass of 258 or 178 Da in fusion proteins. Anal. Biochem. 267, 169–184 (1999).
Acknowledgements
We thank M. Salvati from Bristol-Myers Squibb for the gift of ALRT 1550 and M. Klaus from Roche for the gift of RO 41-5253 and all-trans-4-oxo retinoic acid, P. Eberling for peptide synthesis, B. Blumberg for advice in performing scintillation proximity assays, P. Carpentier for support on ESRF beamline BM14 CRG and C. Schulze-Briese for support on the SLS X065A beamline, J. Cavarelli for help with the ALRT-complex data, and G. Tocchini-Valentini, V. Lamour and P. Hublitz for useful discussions. This work was supported by grants from the Deutsche Forschungsgemeinschaft to R.S. and by funds from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, Université Louis Pasteur de Strasbourg and the Fond National de la Science to the Strasbourg Génopole. We acknowledge the financial support of the European Union for the international research project SPINE. S.S. acknowledges a grant from the CNRS and Eli Lilly.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Stehlin-Gaon, C., Willmann, D., Zeyer, D. et al. All-trans retinoic acid is a ligand for the orphan nuclear receptor RORβ. Nat Struct Mol Biol 10, 820–825 (2003). https://doi.org/10.1038/nsb979
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb979
This article is cited by
-
Cellular and micro-environmental responses influencing the antitumor activity of all-trans retinoic acid in breast cancer
Cell Communication and Signaling (2024)
-
An update on the role and potential mechanisms of clock genes regulating spermatogenesis: A systematic review of human and animal experimental studies
Reviews in Endocrine and Metabolic Disorders (2023)
-
Drug-target interactions prediction using marginalized denoising model on heterogeneous networks
BMC Bioinformatics (2020)
-
Retinoids as an Immunity-modulator in Dermatology Disorders
Archivum Immunologiae et Therapiae Experimentalis (2019)
-
Pharmacological Manipulation of the Circadian Clock: A Possible Approach to the Management of Bipolar Disorder
CNS Drugs (2019)