Abstract
The unprecedented advances in cancer genetics and genomics are rapidly affecting the clinical management of solid tumors. Molecular diagnostics are now an integral part of routine clinical management for patients with lung, colon, and breast cancer. In sharp contrast, molecular biomarkers have been largely excluded from current management algorithms for urologic malignancies. The need for new treatment options that can improve upon the modest outcomes currently associated with muscle-invasive bladder cancer is evident, and validated prognostic molecular biomarkers that can help clinicians to identify patients in need of early, aggressive management are lacking. Robust predictive biomarkers that are able to forecast and stratify responses to emerging targeted therapies are also needed.
Key Points
-
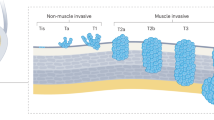
Molecular genetic evidence supports the existence of two distinct pathogenetic pathways for bladder cancer development, corresponding to two distinct biological and clinical phenotypes: non-muscle-invasive and muscle-invasive urothelial carcinoma
-
Disruption to the PI3K–AKT–mTOR pathway and alterations in the tyrosine kinase receptor gene FGFR3 and the oncogene HRAS are associated with non-muscle-invasive bladder cancer
-
The main genetic alterations underlying muscle-invasive bladder cancer involve tumor suppressor genes encoding proteins that regulate cell cycle and apoptosis pathways, including TP53, CDKN2A, CCND1, CDKN1B, and RB1
-
Delineation of the molecular pathways involved in bladder oncogenesis has fueled the quest for much-needed prognostic biomarkers, new targeted therapies, and markers of therapy response
-
A 'molecular grade' (based on FGFR3 and MIB1 expression) and a multitarget fluorescence in situ hybridization (FISH) assay have both achieved sufficient validation to be included in prospective multi-institutional trials
-
A panel of multiple cell cycle control markers—TP53, CDKN1B, CDKN1A, CDKN2A, CCND1, and RBL1—offers superior prognostic power over single marker approaches, and merits further rigorous prospective validation
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mitra, A. P., Datar, R. H. & Cote, R. J. Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J. Clin. Oncol. 24, 5552–5564 (2006).
Mitra, A. P. & Cote, R. J. Molecular screening for bladder cancer: progress and potential. Nat. Rev. Urol. 7, 11–20 (2010).
Mitra, A. P. & Cote, R. J. Molecular pathogenesis and diagnostics of bladder cancer. Ann. Rev. Pathol. 4, 251–285 (2009).
Wu, X. R. Urothelial tumorigenesis: a tale of divergent pathways. Nat. Rev. Cancer 5, 713–725 (2005).
Mo, L. et al. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis. J. Clin. Invest. 117, 314–325 (2007).
Rabbani, F. et al. Prognostic significance of p27(Kip1) expression in bladder cancer. BJU Int. 100, 259–263 (2007).
Sanchez-Carbayo, M., Socci, N. D., Lozano, J., Saint, F. & Cordon-Cardo, C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J. Clin. Oncol. 24, 778–789 (2006).
Sanchez-Carbayo, M. et al. Molecular profiling of bladder cancer using cDNA microarrays: defining histogenesis and biological phenotypes. Cancer Res. 62, 6973–6980 (2002).
Sanchez-Carbayo, M. & Cordon-Cardo, C. Applications of array technology: identification of molecular targets in bladder cancer. Br. J. Cancer 89, 2172–2177 (2003).
Ioachim, E. et al. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha expression in bladder cancer and their associations with other angiogenesis-related proteins. Urol. Int. 77, 255–263 (2006).
Ioachim, E. et al. Thrombospondin-1 expression in urothelial carcinoma: prognostic significance and association with p53 alterations, tumour angiogenesis and extracellular matrix components. BMC Cancer 6, 140 (2006).
Lascombe, I. et al. N-cadherin as a novel prognostic marker of progression in superficial urothelial tumors. Clin. Cancer Res. 12, 2780–2787 (2006).
Rotterud, R., Nesland, J. M., Berner, A. & Fossa, S. D. Expression of the epidermal growth factor receptor family in normal and malignant urothelium. BJU Int. 95, 1344–1350 (2005).
Highshaw, R. A., McConkey, D. J. & Dinney, C. P. Integrating basic science and clinical research in bladder cancer: update from the first bladder Specialized Program of Research Excellence (SPORE). Curr. Opin. Urol. 14, 295–300 (2004).
Clairotte, A. et al. Expression of E-cadherin and alpha-, beta-, gamma-catenins in patients with bladder cancer: identification of gamma-catenin as a new prognostic marker of neoplastic progression in T1 superficial urothelial tumors. Am. J. Clin. Pathol. 125, 119–126 (2006).
Chatterjee, S. J. et al. Combined effects of p53, p21, and pRb expression in the progression of bladder transitional cell carcinoma. J. Clin. Oncol. 22, 1007–1013 (2004).
Beekman, K. W., Bradley, D. & Hussain, M. New molecular targets and novel agents in the treatment of advanced urothelial cancer. Semin. Oncol. 34, 154–164 (2007).
Shariat, S. F., Ashfaq, R., Sagalowsky, A. I. & Lotan, Y. Predictive value of cell cycle biomarkers in nonmuscle invasive bladder transitional cell carcinoma. J. Urol. 177, 481–487 (2007).
Miyamoto, H. et al. Infrequent somatic mutations of the p16 and p15 genes in human bladder cancer: p16 mutations occur only in low-grade and superficial bladder cancers. Oncol. Res. 7, 327–330 (1995).
Miyamoto, H. et al. Analyses of p53 gene mutations in primary human bladder cancer. Oncol. Res. 5, 245–249 (1993).
Birkhahn, M. et al. Predicting recurrence and progression of noninvasive papillary bladder cancer at initial presentation based on quantitative gene expression profiles. Eur. Urol. 57, 12–20 (2010).
Cheng, L. et al. The origins of urothelial carcinoma. Expert Rev. Anticancer Ther. 10, 865–880 (2010).
Mengual, L. et al. Gene expression signature in urine for diagnosing and assessing aggressiveness of bladder urothelial carcinoma. Clin. Cancer Res. 16, 2624–2633 (2010).
Shariat, S. F., Ashfaq, R., Sagalowsky, A. I. & Lotan, Y. Association of cyclin D1 and E1 expression with disease progression and biomarkers in patients with nonmuscle-invasive urothelial cell carcinoma of the bladder. Urol. Oncol. 25, 468–475 (2007).
Stein, J. P. et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J. Clin. Oncol. 19, 666–675 (2001).
Kawauchi, S. et al. 9p21 index as estimated by dual-color fluorescence in situ hybridization is useful to predict urothelial carcinoma recurrence in bladder washing cytology. Hum. Pathol. 40, 1783–1789 (2009).
Kruger, S., Mess, F., Bohle, A. & Feller, A. C. Numerical aberrations of chromosome 17 and the 9p21 locus are independent predictors of tumor recurrence in non-invasive transitional cell carcinoma of the urinary bladder. Int. J. Oncol. 23, 41–48 (2003).
Skacel, M. et al. Multitarget fluorescence in situ hybridization assay detects transitional cell carcinoma in the majority of patients with bladder cancer and atypical or negative urine cytology. J. Urol. 169, 2101–2105 (2003).
Moonen, P. M. et al. UroVysion compared with cytology and quantitative cytology in the surveillance of non-muscle-invasive bladder cancer. Eur. Urol. 51, 1275–80 (2007).
Sarosdy, M. F. et al. Use of a multitarget fluorescence in situ hybridization assay to diagnose bladder cancer in patients with hematuria. J. Urol. 176, 44–47 (2006).
Yoder, B. J. et al. Reflex UroVysion testing of bladder cancer surveillance patients with equivocal or negative urine cytology: a prospective study with focus on the natural history of anticipatory positive findings. Am. J. Clin. Pathol. 127, 295–301 (2007).
Fritsche, H. M. et al. Multicolor FISH (UroVysion) facilitates follow-up of patients with high-grade urothelial carcinoma of the bladder. Am. J. Clin. Pathol. 134, 597–603 (2010).
Karnwal, A. et al. The role of fluorescence in situ hybridization assay for surveillance of non-muscle invasive bladder cancer. Can. J. Urol. 17, 5077–5081 (2010).
Schlomer, B. J., Ho, R., Sagalowsky, A., Ashfaq, R. & Lotan, Y. Prospective validation of the clinical usefulness of reflex fluorescence in situ hybridization assay in patients with atypical cytology for the detection of urothelial carcinoma of the bladder. J. Urol. 183, 62–67 (2010).
Ferra, S. et al. Reflex UroVysion testing in suspicious urine cytology cases. Cancer 117, 7–14 (2009).
Savic, S. et al. The prognostic value of cytology and fluorescence in situ hybridization in the follow-up of nonmuscle-invasive bladder cancer after intravesical bacillus Calmette-Guerin therapy. Int. J. Cancer 124, 2899–2904 (2009).
Maffezzini, M. et al. Prognostic significance of fluorescent in situ hybridisation in the follow-up of non-muscle-invasive bladder cancer. Anticancer Res. 30, 4761–4765 (2010).
Whitson, J., Berry, A., Carroll, P. & Konety, B. A multicolour fluorescence in situ hybridization test predicts recurrence in patients with high-risk superficial bladder tumours undergoing intravesical therapy. BJU Int. 104, 336–339 (2009).
Renshaw, A. A. UroVysion, urine cytology, and the College of American Pathologists: where should we go from here? Arch. Pathol. Lab. Med. 134, 1106–1107 (2010).
Lopez-Knowles, E. et al. PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors. Cancer Res. 66, 7401–7404 (2006).
Kompier, L. C. et al. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS ONE 5, e13821 (2010).
van Rhijn, B. W. et al. Molecular grade (FGFR3/MIB-1) and EORTC risk scores are predictive in primary non-muscle-invasive bladder cancer. Eur. Urol. 58, 433–441 (2010).
Mason, R. A. et al. EGFR pathway polymorphisms and bladder cancer susceptibility and prognosis. Carcinogenesis 30, 1155–1160 (2009).
Simonetti, S. et al. Role of polysomy 17 in transitional cell carcinoma of the bladder: immunohistochemical study of HER2/neu expression and fish analysis of c-erbB-2 gene and chromosome 17. Int. J. Surg. Pathol. 17, 198–205 (2009).
Latif, Z. et al. HER2/neu gene amplification and protein overexpression in G3 pT2 transitional cell carcinoma of the bladder: a role for anti-HER2 therapy? Eur. J. Cancer 40, 56–63 (2004).
Gandour-Edwards, R. et al. Does HER2/neu expression provide prognostic information in patients with advanced urothelial carcinoma? Cancer 95, 1009–1015 (2002).
Eissa, S., Ali, H. S., Al Tonsi, A. H., Zaglol, A. & El Ahmady, O. HER2/neu expression in bladder cancer: relationship to cell cycle kinetics. Clin. Biochem. 38, 142–148 (2005).
Billerey, C. et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am. J. Pathol. 158, 1955–1959 (2001).
Leibl, S. et al. EGFR expression in urothelial carcinoma of the upper urinary tract is associated with disease progression and metaplastic morphology. APMIS 116, 27–32 (2008).
Bolenz, C. et al. Human epidermal growth factor receptor 2 expression status provides independent prognostic information in patients with urothelial carcinoma of the urinary bladder. BJU Int. 106, 1216–1222 (2010).
Zuiverloon, T. C. et al. Fibroblast growth factor receptor 3 mutation analysis on voided urine for surveillance of patients with low-grade non-muscle-invasive bladder cancer. Clin. Cancer Res. 16, 3011–3018 (2010).
Miyake, M. et al. Fibroblast growth factor receptor 3 mutation in voided urine is a useful diagnostic marker and significant indicator of tumor recurrence in non-muscle invasive bladder cancer. Cancer Sci. 101, 250–258 (2010).
Hernandez, S. et al. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J. Clin. Oncol. 24, 3664–3671 (2006).
Bolenz, C. & Lotan, Y. Translational research in bladder cancer: from molecular pathogenesis to useful tissue biomarkers. Cancer Biol. Ther. 10, 407–415 (2010).
Chakravarti, A. et al. Expression of the epidermal growth factor receptor and Her-2 are predictors of favorable outcome and reduced complete response rates, respectively, in patients with muscle-invading bladder cancers treated by concurrent radiation and cisplatin-based chemotherapy: a report from the Radiation Therapy Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 62, 309–317 (2005).
Jimenez, R. E. et al. Her-2/neu overexpression in muscle-invasive urothelial carcinoma of the bladder: prognostic significance and comparative analysis in primary and metastatic tumors. Clin. Cancer Res. 7, 2440–2447 (2001).
Ravery, V. et al. Evaluation of epidermal growth factor receptor, transforming growth factor alpha, epidermal growth factor and c-erbB2 in the progression of invasive bladder cancer. Urol. Res. 25, 9–17 (1997).
Sarkis, A. S. et al. Nuclear overexpression of p53 protein in transitional cell bladder carcinoma: a marker for disease progression. J. Natl Cancer Inst. 85, 53–59 (1993).
Sarkis, A. S. et al. Association of P53 nuclear overexpression and tumor progression in carcinoma in situ of the bladder. J. Urol. 152, 388–392 (1994).
Sarkis, A. S. et al. Prognostic value of p53 nuclear overexpression in patients with invasive bladder cancer treated with neoadjuvant MVAC. J. Clin. Oncol. 13, 1384–1390 (1995).
Shariat, S. F. et al. p53 expression in patients with advanced urothelial cancer of the urinary bladder. BJU Int. 105, 489–495 (2010).
Lopez-Beltran, A. et al. Cyclin D3 expression in primary Ta/T1 bladder cancer. J. Pathol. 209, 106–113 (2006).
Lopez-Beltran, A. et al. Prognostic factors in stage T1 grade 3 bladder cancer survival: the role of G1-S. modulators (p53, p21Waf1, p27kip1, Cyclin D1, and Cyclin D3) and proliferation index (ki67-MIB1). Eur. Urol. 45, 606–612 (2004).
Fu, T. Y., Tu, M. S., Tseng, H. H. & Wang, J. S. Overexpression of p27kip1 in urinary bladder urothelial carcinoma. Int. J. Urol. 14, 1084–1087 (2007).
Yin, M., Bastacky, S., Parwani, A. V., McHale, T. & Dhir, R. P16ink4 Immunoreactivity is a reliable marker for urothelial carcinoma in situ. Hum. Pathol. 39, 527–535 (2008).
Kruger, S., Mahnken, A., Kausch, I. & Feller, A. C. P16 immunoreactivity is an independent predictor of tumor progression in minimally invasive urothelial bladder carcinoma. Eur. Urol. 47, 463–467 (2005).
Sgambato, A. et al. Loss of P27Kip1 expression correlates with tumor grade and with reduced disease-free survival in primary superficial bladder cancers. Cancer Res. 59, 3245–3250 (1999).
Garcia del Muro, X. et al. p53 and p21 Expression levels predict organ preservation and survival in invasive bladder carcinoma treated with a combined-modality approach. Cancer 100, 1859–1867 (2004).
Shariat, S. F. et al. Multiple biomarkers improve prediction of bladder cancer recurrence and mortality in patients undergoing cystectomy. Cancer 112, 315–325 (2008).
Shariat, S. F. et al. Predictive value of combined immunohistochemical markers in patients with pT1 urothelial carcinoma at radical cystectomy. J. Urol. 182, 78–84 (2009).
Shariat, S. F., Ashfaq, R., Sagalowsky, A. I. & Lotan, Y. Predictive value of cell cycle biomarkers in nonmuscle invasive bladder transitional cell carcinoma. J. Urol. 177, 481–487 (2007).
Dalbagni, G. et al. Molecular genetic alterations of chromosome 17 and p53 nuclear overexpression in human bladder cancer. Diagn. Mol. Pathol. 2, 4–13 (1993).
Nishiyama, N. et al. Genome-wide DNA methylation profiles in urothelial carcinomas and urothelia at the precancerous stage. Cancer Sci. 101, 231–240 (2010).
Lin, H. H. et al. Increase sensitivity in detecting superficial, low grade bladder cancer by combination analysis of hypermethylation of E-cadherin, p16, p14, RASSF1A genes in urine. Urol. Oncol. 28, 597–602 (2010).
Vinci, S. et al. Quantitative methylation analysis of BCL2, hTERT, and DAPK promoters in urine sediment for the detection of non-muscle-invasive urothelial carcinoma of the bladder: a prospective, two-center validation study. Urol. Oncol. 29, 150–156 (2009).
Cabello, M. J. et al. Multiplexed methylation profiles of tumor suppressor genes in bladder cancer. J. Mol. Diagn. 13, 29–40 (2011).
Vallot, C. et al. A novel epigenetic phenotype associated with the most aggressive pathway of bladder tumor progression. J. Natl Cancer Inst. 103, 47–60 (2011).
Dudziec, E. et al. Hypermethylation of CpG islands and shores around specific microRNAs and mirtrons is associated with the phenotype and presence of bladder cancer. Clin. Cancer Res. 17, 1287–1296 (2011).
Wiklund, E. D. et al. Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. Int. J. Cancer 128, 1327–1334 (2011).
Serizawa, R. R. et al. Integrated genetic and epigenetic analysis of bladder cancer reveals an additive diagnostic value of FGFR3 mutations and hypermethylation events. Int. J. Cancer 129, 78–87 (2011).
Catto, J. W. et al. Promoter hypermethylation is associated with tumor location, stage, and subsequent progression in transitional cell carcinoma. J. Clin. Oncol. 23, 2903–2910 (2005).
Friedrich, M. G. et al. Detection of methylated apoptosis-associated genes in urine sediments of bladder cancer patients. Clin. Cancer Res. 10, 7457–7465 (2004).
Chan, M. W. et al. Hypermethylation of multiple genes in tumor tissues and voided urine in urinary bladder cancer patients. Clin. Cancer Res. 8, 464–470 (2002).
Yates, D. R. et al. Methylational urinalysis: a prospective study of bladder cancer patients and age stratified benign controls. Oncogene 25, 1984–1988 (2006).
Hoque, M. O. et al. Quantitation of promoter methylation of multiple genes in urine DNA and bladder cancer detection. J. Natl Cancer Inst. 98, 996–1004 (2006).
Yates, D. R. et al. Promoter hypermethylation identifies progression risk in bladder cancer. Clin. Cancer Res. 13, 2046–2053 (2007).
Cheng, L. et al. Bladder cancer: translating molecular genetic insights into clinical practice. Hum. Pathol. 42, 455–481 (2011).
Miyamoto, H. et al. Low-grade papillary urothelial carcinoma of the urinary bladder: a clinicopathologic analysis of a post-World Health Organization/International Society of Urological Pathology classification cohort from a single academic center. Arch. Pathol. Lab. Med. 134, 1160–1163 (2010).
Mitra, A. P. et al. Generation of a concise gene panel for outcome prediction in urinary bladder cancer. J. Clin. Oncol. 27, 3929–3937 (2009).
Rothman, N. et al. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat. Genet. 42, 978–984 (2010).
Sanchez-Carbayo, M. & Cordon-Cardo, C. Molecular alterations associated with bladder cancer progression. Semin. Oncol. 34, 75–84 (2007).
Smith, S. C. et al. A 20-gene model for molecular nodal staging of bladder cancer: development and prospective assessment. Lancet Oncol. 12, 137–143 (2011).
van Rhijn, B. W. et al. Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB-1 is superior to pathologic grade for the prediction of clinical outcome. J. Clin. Oncol. 21, 1912–1921 (2003).
Sylvester, R. J. et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 49, 466–477 (2006).
Lindgren, D. et al. Combined gene expression and genomic profiling define two intrinsic molecular subtypes of urothelial carcinoma and gene signatures for molecular grading and outcome. Cancer Res. 70, 3463–3472 (2010).
Quintero, A. et al. Ki-67 MIB1 labelling index and the prognosis of primary TaT1 urothelial cell carcinoma of the bladder. J. Clin. Pathol. 59, 83–88 (2006).
Margulis, V., Shariat, S. F., Ashfaq, R., Sagalowsky, A. I. & Lotan, Y. Ki-67 is an independent predictor of bladder cancer outcome in patients treated with radical cystectomy for organ-confined disease. Clin. Cancer Res. 12, 7369–7373 (2006).
Margulis, V. et al. Multi-institutional validation of the predictive value of Ki-67 labeling index in patients with urinary bladder cancer. J. Natl Cancer Inst. 101, 114–119 (2009).
Ramos, D. et al. Prognostic value of morphometry in low grade papillary urothelial bladder neoplasms. Anal. Quant. Cytol. Histol. 26, 285–294 (2004).
Ali-El-Dein, B. et al. Superficial bladder tumours: analysis of prognostic factors and construction of a predictive index. BJU Int. 92, 393–399 (2003).
Loughman, N. T., Lin, B. P., Dent, O. F. & Newland, R. C. DNA ploidy of bladder cancer using bladder biopsy supernate specimens. Anal. Quant. Cytol. Histol. 25, 146–158 (2003).
Baak, J. P. et al. DNA cytometric features in biopsies of TaT1 urothelial cell cancer predict recurrence and stage progression more accurately than stage, grade, or treatment modality. Urology 61, 1266–1272 (2003).
Bol, M. G. et al. Correlation of grade of urothelial cell carcinomas and DNA histogram features assessed by flow cytometry and automated image cytometry. Anal. Cell. Pathol. 25, 147–153 (2003).
Bellaoui, H. et al. Flow cytometric DNA analysis and cytology in diagnosis and prognosis of bladder tumors: preliminary results of a comparative study of bladder lavage. Ann. Urol. (Paris) 36, 45–52 (2002).
Caraway, N. P., Khanna, A., Payne, L., Kamat, A. M. & Katz, R. L. Combination of cytologic evaluation and quantitative digital cytometry is reliable in detecting recurrent disease in patients with urinary diversions. Cancer 111, 323–329 (2007).
Falkman, K., Tribukait, B., Nyman, C. R., Larsson, P. & Norming, U. S-phase fraction in superficial urothelial carcinoma of the bladder—a prospective, long-term, follow-up study. Scand. J. Urol. Nephrol. 38, 278–284 (2004).
Lin, J. et al. E-cadherin promoter polymorphism (C-160A) and risk of recurrence in patients with superficial bladder cancer. Clin. Genet. 70, 240–245 (2006).
Palit, V., Phillips, R. M., Puri, R., Shah, T. & Bibby, M. C. Expression of HIF-1alpha and Glut-1 in human bladder cancer. Oncol. Rep. 14, 909–913 (2005).
Chai, C. Y. et al. Hypoxia-inducible factor-1alpha expression correlates with focal macrophage infiltration, angiogenesis and unfavourable prognosis in urothelial carcinoma. J. Clin. Pathol. 61, 658–664 (2008).
Crew, J. P. et al. Urinary vascular endothelial growth factor and its correlation with bladder cancer recurrence rates. J. Urol. 161, 799–804 (1999).
Crew, J. P. et al. Vascular endothelial growth factor is a predictor of relapse and stage progression in superficial bladder cancer. Cancer Res. 57, 5281–5285 (1997).
Turner, K. J. et al. The hypoxia-inducible genes VEGF and CA9 are differentially regulated in superficial versus invasive bladder cancer. Br. J. Cancer 86, 1276–1282 (2002).
Tickoo, S. K. et al. Hypoxia-inducible factor and mammalian target of rapamycin pathway markers in urothelial carcinoma of the bladder: possible therapeutic implications. BJU Int. 107, 844–849 (2011).
Platt, F. M. et al. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin. Cancer Res. 15, 6008–6017 (2009).
Schultz, L. et al. Expression status and prognostic significance of mammalian target of rapamycin pathway members in urothelial carcinoma of urinary bladder after cystectomy. Cancer 116, 5517–5526 (2010).
Comperat, E. et al. Aurora-A/STK-15 is a predictive factor for recurrent behaviour in non-invasive bladder carcinoma: a study of 128 cases of non-invasive neoplasms. Virchows Arch. 450, 419–424 (2007).
Mhawech-Fauceglia, P., Fischer, G., Beck, A., Cheney, R. T. & Herrmann, F. R. Raf1, Aurora-A/STK15 and E-cadherin biomarkers expression in patients with pTa/pT1 urothelial bladder carcinoma; a retrospective TMA study of 246 patients with long-term follow-up. Eur. J. Surg. Oncol. 32, 439–444 (2006).
Veerla, S., Panagopoulos, I., Jin, Y., Lindgren, D. & Hoglund, M. Promoter analysis of epigenetically controlled genes in bladder cancer. Genes Chromosomes Cancer 47, 368–378 (2008).
Wszolek, M. F. et al. A MicroRNA expression profile defining the invasive bladder tumor phenotype. Urol. Oncol. http://dx.doi.org/10.1016/j.urolonc.2009.08.024 (2009).
Catto, J. W. et al. Distinct microRNA alterations characterize high- and low-grade bladder cancer. Cancer Res. 69, 8472–8481 (2009).
Iyer, G., Milowsky, M. I. & Bajorin, D. F. Novel strategies for treating relapsed/refractory urothelial carcinoma. Expert Rev. Anticancer Ther. 10, 1917–1932 (2010).
Wallerand, H., Reiter, R. R. & Ravaud, A. Molecular targeting in the treatment of either advanced or metastatic bladder cancer or both according to the signalling pathways. Curr. Opin. Urol. 18, 524–532 (2008).
Amit, D., Tamir, S., Birman, T., Gofrit, O. N. & Hochberg, A. Development of targeted therapy for bladder cancer mediated by a double promoter plasmid expressing diphtheria toxin under the control of IGF2-P3 and IGF2-P4 regulatory sequences. Int. J. Clin. Exp. Med. 4, 91–102 (2011).
Gerullis, H. et al. Long-term response in advanced bladder cancer involving the use of temsirolimus and vinflunine after platin resistance. Anticancer Drugs 22, 940–943 (2011).
Yafi, F. A., North, S. & Kassouf, W. First- and second-line therapy for metastatic urothelial carcinoma of the bladder. Curr. Oncol. 18, e25–34 (2011).
Zhang, X. & Godbey, W. T. Preclinical evaluation of a gene therapy treatment for transitional cell carcinoma. Cancer Gene Ther. 18, 34–41 (2011).
Smaldone, M. C. & Davies, B. J. BC-819, a plasmid comprising the H19 gene regulatory sequences and diphtheria toxin A, for the potential targeted therapy of cancers. Curr. Opin. Mol. Ther. 12, 607–616 (2010).
Kramer, M. W., Krege, S., Peters, I., Merseburger, A. S. & Kuczyk, M. A. Targeted therapy of urological tumours. Experimental field or established therapeutic approach? Urologe A. 49, 1260–1265 (2010).
Ching, C. B. & Hansel, D. E. Expanding therapeutic targets in bladder cancer: the PI3K/Akt/mTOR pathway. Lab. Invest. 90, 1406–1414 (2010).
Black, P. C., Agarwal, P. K. & Dinney, C. P. Targeted therapies in bladder cancer—an update. Urol. Oncol. 25, 433–438 (2007).
Black, P. C. & Dinney, C. P. Bladder cancer angiogenesis and metastasis--translation from murine model to clinical trial. Cancer Metastasis Rev. 26, 623–634 (2007).
Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 3, 11–22 (2003).
Bellmunt, J., Hussain, M. & Dinney, C. P. Novel approaches with targeted therapies in bladder cancer. Therapy of bladder cancer by blockade of the epidermal growth factor receptor family. Crit. Rev. Oncol. Hematol. 46, S85–S104 (2003).
Wallerand, H., Robert, G., Bernhard, J. C., Ravaud, A. & Ferriere, J. M. Targeted therapy for locally advanced and/or metastatic bladder cancer. Prog. Urol. 18, 407–417 (2008).
Hussain, M. H. et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J. Clin. Oncol. 25, 2218–2224 (2007).
Hansel, D. E., Swain, E., Dreicer, R. & Tubbs, R. R. HER2 overexpression and amplification in urothelial carcinoma of the bladder is associated with MYC coamplification in a subset of cases. Am. J. Clin. Pathol. 130, 274–281 (2008).
Inoue, K. et al. Paclitaxel enhances the effects of the anti-epidermal growth factor receptor monoclonal antibody ImClone C225 in mice with metastatic human bladder transitional cell carcinoma. Clin. Cancer Res. 6, 4874–4884 (2000).
Perrotte, P. et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin. Cancer Res. 5, 257–265 (1999).
Philips, G. K. et al. A phase II trial of cisplatin, fixed dose-rate gemcitabine and gefitinib for advanced urothelial tract carcinoma: results of the Cancer and Leukaemia Group B 90102. BJU Int. 101, 20–25 (2008).
Philips, G. K. et al. A phase II trial of cisplatin (C), gemcitabine (G.) and gefitinib for advanced urothelial tract carcinoma: results of Cancer and Leukemia Group B (CALGB) 90102. Ann. Oncol. 20, 1074–1079 (2009).
Wulfing, C. et al. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer 115, 2881–2890 (2009).
Bradley, D. A. et al. Randomized, double-blind, placebo-controlled phase II trial of maintenance sunitinib versus placebo after chemotherapy for patients with advanced urothelial carcinoma: scientific rationale and study design. Clin. Genitourin. Cancer 5, 460–463 (2007).
Hahn, N. M. et al. Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04–75. J. Clin. Oncol. 29, 1525–1530 (2011).
Elfiky, A. A. & Rosenberg, J. E. Targeting angiogenesis in bladder cancer. Curr. Oncol. Rep. 11, 244–249 (2009).
Miyamoto, H. et al. Non-invasive papillary urothelial neoplasms: the 2004 WHO/ISUP classification system. Pathol. Int. 60, 1–8 (2010).
Millan-Rodriguez, F., Chechile-Toniolo, G., Salvador-Bayarri, J., Palou, J. & Vicente-Rodriguez, J. Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J. Urol. 163, 73–78 (2000).
Soloway, M. S., Sofer, M. & Vaidya, A. Contemporary management of stage T1 transitional cell carcinoma of the bladder. J. Urol. 167, 1573–1583 (2002).
Bensalah, K., Montorsi, F. & Shariat, S. F. Challenges of cancer biomarker profiling. Eur. Urol. 52, 1601–1609 (2007).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Netto, G. Molecular biomarkers in urothelial carcinoma of the bladder: are we there yet?. Nat Rev Urol 9, 41–51 (2012). https://doi.org/10.1038/nrurol.2011.193
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2011.193
This article is cited by
-
Promoter hypomethylation as potential confounder of Ras gene overexpression and their clinical significance in subsets of urothelial carcinoma of bladder
Molecular Biology Reports (2021)
-
MicroRNAs and target molecules in bladder cancer
Medical Oncology (2020)
-
Performance of novel non-invasive urine assay UroSEEK in cohorts of equivocal urine cytology
Virchows Archiv (2020)
-
Cell Cycle Markers in the Evaluation of Bladder Cancer
Pathology & Oncology Research (2020)
-
Organ preservation in bladder cancer: an opportunity for truly personalized treatment
Nature Reviews Urology (2019)