Abstract
Many patients with interstitial cystitis (IC) find that particular foods exacerbate disease symptoms. These patients may modify their diet to manage symptoms, but the mechanism by which dietary modification benefits patients with IC is unclear. We hypothesize that integration of neural signals from pelvic organs mediates the effects of diet on symptoms of IC. In animal models, pelvic inflammation is subject to crosstalk, so an inflammatory stimulus in one pelvic organ evokes a response in an independent organ. Recent data show that the colon can modulate bladder-associated pelvic pain in mice. As pelvic organs are innervated through shared circuitry, perceived pelvic pain might occur when spatial summation of individual pelvic inputs exceeds a threshold. Through this mechanism, a noxious dietary stimulus, which otherwise does not exceed the pain threshold in a normal individual, may substantially exacerbate pain in a patient with bladder symptoms. Repeated painful stimuli over time further contribute to symptoms by a process of temporal summation, resulting in enhanced responsiveness through central sensitization. Thus, pelvic organ crosstalk might modulate symptoms of pelvic pain by spatial and temporal summation, suggesting a mechanism for the benefits of dietary modification in patients with IC, as well as therapeutic opportunities.
Key Points
-
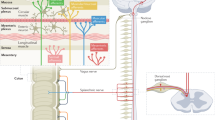
Animal studies have shown evidence of neurophysiological crosstalk between the bladder and bowel due to shared spinal and pelvic nerve circuitry; this communication may both positively and negatively modulate bladder-associated pelvic pain
-
Disparate regional stimuli can be integrated by spatial summation, and repetitive stimuli are integrated by temporal summation; therefore, we hypothesize that pain in interstitial cystitis (IC) is subject to summation by these mechanisms, resulting in exacerbation of bladder symptoms by dietary influences and time-dependent wind-up
-
Summation between a strong bladder pain signal and an otherwise subthreshold noxious dietary stimulus in the gastrointestinal tract may explain food sensitivities in patients with IC
-
The gastrointestinal tract and pelvic dermatome have the potential to be new sites for therapeutic intervention for pain symptoms in patients with IC
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hanno P (2007) Interstitial cystitis and related diseases. In Campbell-Walsh Urology, edn 9, 631–670 (Eds Wein A et al.) Philadelphia: WB Saunders
Theoharides TC et al. (1998) Interstitial cystitis: a neuroimmunoendocrine disorder. Ann NY Acad Sci 840: 619–634
Koziol JA et al. (1993) The natural history of interstitial cystitis: a survey of 374 patients. J Urol 149: 465–469
Shorter B et al. (2007) Effect of comestibles on symptoms of interstitial cystitis. J Urol 178: 145–152
Clemens JQ et al. (2006) Predictors of symptom severity in patients with chronic prostatitis and interstitial cystitis. J Urol 175: 963–966
Rudick CN et al. (2007) Organ cross talk modulates pelvic pain. Am J Physiol Regul Integr Comp Physiol 293: R1191–R1198
Ustinova EE et al. (2007) Sensitization of pelvic nerve afferents and mast cell infiltration in the urinary bladder following chronic colonic irritation is mediated by neuropeptides. Am J Physiol Renal Physiol 292: 123–130
Floyd K et al. (1982) Inhibitory interactions between colonic and vesical afferents in the micturition reflex of the cat. J Physiol 322: 45–52
McMahon SB and Morrison JF (1982) Two group of spinal interneurones that respond to stimulation of the abdominal viscera of the cat. J Physiol 322: 21–34
Berkley KJ (2005) A life of pelvic pain. Physiol Behav 86: 272–280
Berkley KJ et al. (2005) The pains of endometriosis. Science 308: 1587–1589
Alagiri M et al. (1997) Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology 49: 52–57
Winnard KP et al. (2006) Cross-organ interactions between reproductive, gastrointestinal, and urinary tracts: modulation by estrous stage and involvement of the hypogastric nerve. Am J Physiol Regul Integr Comp Physiol 291: 1592–1601
Chen MC et al. (2006) Tumor necrosis factor promotes differential trafficking of bladder mast cells in neurogenic cystitis. J Urol 175: 754–759
Chen MC et al. (2007) RANTES mediates TNF-dependent lamina propria mast cell accumulation and barrier dysfunction in neurogenic cystitis. Am J Physiol Renal Physiol 292: 1372–1379
Chen MC et al. (2006) Urothelial lesion formation is mediated by TNFR1 during neurogenic cystitis. Am J Physiol Renal Physiol 291: 741–749
Tomaszewski JE et al. (2001) Biopsy features are associated with primary symptoms in interstitial cystitis: results from the interstitial cystitis database study. Urology 57: 67–81
Leiby BE et al. (2007) Discovery of morphological subgroups that correlate with severity of symptoms in interstitial cystitis: a proposed biopsy classification system. J Urol 177: 142–148
Staud R et al. (2007) Spatial summation of mechanically evoked muscle pain and painful aftersensations in normal subjects and fibromyalgia patients. Pain 130: 177–187
Defrin R et al. (2003) Spatial summation of pressure pain: effect of body region. Pain 106: 471–480
Price DD and Staud R (2005) Neurobiology of fibromyalgia syndrome. J Rheumatol Suppl 75: 22–28
Staud R et al. (2003) Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain 102: 87–95
Staud R et al. (2001) Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain 91: 165–175
Wesselmann U (2001) Neurogenic inflammation and chronic pelvic pain. World J Urol 19: 180–185
Wesselmann U (2001) Interstitial cystitis: a chronic visceral pain syndrome. Urology 57: 32–39
McCormick NB (1999) When pleasure causes pain: living with interstitial cystitis. Sex Disabil 17: 7–18
Rudick CN et al. (2008) Experimental autoimmune prostatitis induces chronic pelvic pain. Am J Physiol Regul Integr Comp Physiol 294: 1268–1275
Julius D and Basbaum AI (2001) Molecular mechanisms of nociception. Nature 413: 203–210
Elbadawi A (1997) Interstitial cystitis: a critique of current concepts with a new proposal for pathologic diagnosis and pathogenesis. Urology 49: 14–40
D'Andrea MR et al. (2005) Lack of neurokinin-1 receptor expression affects tissue mast cell numbers but not their spatial relationship with nerves. Am J Physiol Regul Integr Comp Physiol 288: 491–500
Rudick CN et al. (2008) Mast cell-derived histamine mediates cystitis pain. PLoS ONE 3: e2096
Mukerji G (2006) Transient receptor potential vanilloid receptor subtype 1 in painful bladder syndrome and its correlation with pain. J Urol 176: 797–801
Woolf CJ and Salter MW (2000) Neuronal plasticity: increasing the gain in pain. Science 288: 1765–1769
Apkarian AV et al. (2004) Chronic pain patients are impaired on an emotional decision-making task. Pain 108: 129–136
Apkarian AV et al. (2004) Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 24: 10410–10415
Pukall CF et al. (2006) Tender point examination in women with vulvar vestibulitis syndrome. Clin J Pain 22: 601–609
Tripp DA et al. (2006) Catastrophizing and pain-contingent rest predict patient adjustment in men with chronic prostatitis/chronic pelvic pain syndrome. J Pain 7: 697–708
Thilagarajah R et al. (2001) Oral cimetidine gives effective symptom relief in painful bladder disease: a prospective, randomized, double-blind placebo-controlled trial. BJU Int 87: 207–212
Parsons CL et al. (1998) The role of urinary potassium in the pathogenesis and diagnosis of interstitial cystitis. J Urol 159: 1862–1866
Parsons CL (2006) Advances in the treatment of interstitial cystitis. Expert Opin Pharmacother 7: 411–419
Binshtok AM et al. (2007) Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 449: 607–610
Verne GN et al. (2003) Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain 105: 223–230
Verne GN et al. (2005) Intrarectal lidocaine is an effective treatment for abdominal pain associated with diarrhea-predominant irritable bowel syndrome. J Pain 6: 493–496
Geha PY et al. (2007) Brain activity for spontaneous pain of postherpetic neuralgia and its modulation by lidocaine patch therapy. Pain 128: 88–100
Hines R et al. (2002) Use of lidocaine patch 5% for chronic low back pain: a report of four cases. Pain Med 3: 361–365
Bleakman D et al. (2006) Glutamate receptors and pain. Semin Cell Dev Biol 17: 592–604
Millecamps M et al. (2007) [D]-cycloserine reduces neuropathic pain behavior through limbic NMDA-mediated circuitry. Pain 132: 108–123
Sills GJ (2006) The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol 6: 108–113
Crofford LJ et al. (2005) Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 52: 1264–1273
Richter RW et al. (2005) Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain 6: 253–260
Acknowledgements
We thank Dr Anthony Schaeffer and Dr Praveen Thumbikat for many helpful discussions, and Dr Thumbikat for coining the term “Pepperoni hypothesis”. This work was supported by NIDDK awards R01DK066112 (DJ Klumpp) and T32DK062716-05 (CN Rudick).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Klumpp, D., Rudick, C. Summation model of pelvic pain in interstitial cystitis. Nat Rev Urol 5, 494–500 (2008). https://doi.org/10.1038/ncpuro1203
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpuro1203
This article is cited by
-
Sexual pain and IC/BPS in women
BMC Urology (2019)
-
Does central sensitization help explain idiopathic overactive bladder?
Nature Reviews Urology (2016)
-
Stool-based biomarkers of interstitial cystitis/bladder pain syndrome
Scientific Reports (2016)