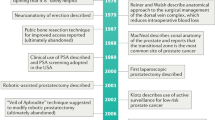
Key Points
-
Patients with high-risk penile cancer tumours and without palpable lymphadenopathy benefit from inguinal lymph node dissection, as many of these men harbour occult metastasis
-
Modified and superficial inguinal lymph node dissection and dynamic sentinel lymph node sampling, as well as minimally invasive techniques, have been developed to decrease the morbidity of traditional radical inguinal lymphadenectomy
-
Patients with large or bulky nodal metastasis benefit from neoadjuvant chemotherapy before surgical intervention
-
High-level evidence of a benefit of radiotherapy in patients with penile cancer with lymph node metastasis is currently lacking
-
Targeted therapies that have demonstrated efficacy in squamous cell carcinomas of other sites might also be effective in treating men with penile cancer and nodal metastasis and advanced disease
Abstract
Penile cancer is a rare disease that causes considerable physical and psychological patient morbidity, especially at advanced stages. Patients with low-stage nodal metastasis can achieve durable survival with surgery alone, but those with extensive locoregional metastasis have overall low survival. Contemporary management strategies for lymph node involvement in penile cancer aim to minimize the morbidity associated with traditional radical inguinal lymphadenectomy through appropriate risk stratification while optimizing oncological outcomes. Modified (or superficial) inguinal lymph node dissection and dynamic sentinel lymph node biopsy are diagnostic modalities that have been recommended in patients with high-risk primary penile tumours and nonpalpable inguinal lymph nodes. In addition, advances in minimally invasive and robot-assisted lymphadenectomy techniques are being investigated in patients with penile cancer and might further decrease lymphadenectomy-related adverse effects. The management of patients with advanced disease has evolved to include multimodal treatment with systemic chemotherapy before surgical intervention and can include adjuvant chemotherapy after pelvic lymphadenectomy. The role of radiotherapy in the neoadjuvant or adjuvant setting remains largely unclear, owing to a lack of high-level evidence of possible benefits. New targeted therapies have shown efficacy in squamous cell carcinomas of other sites and might also prove effective in patients with penile cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kieffer, J. M. et al. Quality of life for patients treated for penile cancer. J. Urol. 192, 1105–1110 (2014).
Siegel, R., Ma, J., Zou, Z. & Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 64, 9–29 (2014).
Ornellas, A. A. Management of penile cancer. J. Surg. Oncol. 97, 199–200 (2008).
Christodoulidou, M., Sahdev, V., Houssein, S. & Muneer, A. Epidemiology of penile cancer. Curr. Probl. Cancer 39, 126–136 (2015).
Downes, M. R. Review of in situ and invasive penile squamous cell carcinoma and associated non-neoplastic dermatological conditions. J. Clin. Pathol. 68, 333–340 (2015).
Diorio, G. J., Leone, A. R. & Spiess, P. E. Management of penile cancer. Urology 96, 15–21 (2016).
Larke, N. L., Thomas, S. L., dos Santos Silva, I. & Weiss, H. A. Male circumcision and penile cancer: a systematic review and meta-analysis. Cancer Causes Control 22, 1097–1110 (2011).
Gursel, E. O., Georgountzos, C., Uson, A. C., Melicow, M. M. & Veenema, R. J. Penile cancer. Urology 1, 569–578 (1973).
Srinivas, V., Morse, M. J., Herr, H. W., Sogani, P. C. & Whitmore, W. F. Jr. Penile cancer: relation of extent of nodal metastasis to survival. J. Urol. 137, 880–882 (1987).
Hakenberg, O. W. et al. EAU guidelines on penile cancer: 2014 update. Eur. Urol. 67, 142–150 (2015).
Ficarra, V., Akduman, B., Bouchot, O., Palou, J. & Tobias-Machado, M. Prognostic factors in penile cancer. Urology 76, S66–S73 (2010).
Pandey, D., Mahajan, V. & Kannan, R. R. Prognostic factors in node-positive carcinoma of the penis. J. Surg. Oncol. 93, 133–138 (2006).
Kroon, B. K. et al. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. J. Urol. 173, 816–819 (2005).
McDougal, W. S. Carcinoma of the penis: improved survival by early regional lymphadenectomy based on the histological grade and depth of invasion of the primary lesion. J. Urol. 154, 1364–1366 (1995).
Ornellas, A. A. et al. Surgical treatment of invasive squamous cell carcinoma of the penis: Brazilian National Cancer Institute long-term experience. J. Surg. Oncol. 97, 487–495 (2008).
Stephenson, A. J. & Sheinfeld, J. The role of retroperitoneal lymph node dissection in the management of testicular cancer. Urol. Oncol. 22, 225–233 (2004).
Djajadiningrat, R. S., van Werkhoven, E. & Horenblas, S. Prophylactic pelvic lymph node dissection in patients with penile cancer. J. Urol. 193, 1976–1980 (2015).
Spiess, P. E., Hernandez, M. S. & Pettaway, C. A. Contemporary inguinal lymph node dissection: minimizing complications. World J. Urol. 27, 205–212 (2009).
Clark, P. E. et al. Penile cancer: clinical practice guidelines in oncology. J. Natl Compr. Canc. Netw. 11, 594–615 (2013).
Sacco, A. G. & Cohen, E. E. Current treatment options for recurrent or metastatic head and neck squamous cell carcinoma. J. Clin. Oncol. 33, 3305–3313 (2015).
Leijte, J. A., Kirrander, P., Antonini, N., Windahl, T. & Horenblas, S. Recurrence patterns of squamous cell carcinoma of the penis: recommendations for follow-up based on a two-centre analysis of 700 patients. Eur. Urol. 54, 161–168 (2008).
Ranganath, R., Singh, S. S. & Sateeshan, B. Sarcomatoid carcinoma of the penis: clinicopathologic features. Indian J. Urol. 24, 267–268 (2008).
Pizzocaro, G. et al. EAU penile cancer guidelines 2009. Eur. Urol. 57, 1002–1012 (2010).
Kirrander, P., Andren, O. & Windahl, T. Dynamic sentinel node biopsy in penile cancer: initial experiences at a Swedish referral centre. BJU Int. 111, E48–E53 (2013).
Graafland, N. M. et al. Prognostic factors for occult inguinal lymph node involvement in penile carcinoma and assessment of the high-risk EAU subgroup: a two-institution analysis of 342 clinically node-negative patients. Eur. Urol. 58, 742–747 (2010).
Campos, R. S., Lopes, A., Guimaraes, G. C., Carvalho, A. L. & Soares, F. A. E-Cadherin, MMP-2, and MMP-9 as prognostic markers in penile cancer: analysis of 125 patients. Urology 67, 797–802 (2006).
Zhu, Y. et al. The value of squamous cell carcinoma antigen in the prognostic evaluation, treatment monitoring and followup of patients with penile cancer. J. Urol. 180, 2019–2023 (2008).
Chaux, A. et al. The prognostic index: a useful pathologic guide for prediction of nodal metastases and survival in penile squamous cell carcinoma. Am. J. Surg. Pathol. 33, 1049–1057 (2009).
Edge, S. et al. (eds) AJCC Cancer Staging Manual 7th edn (Springer, 2010).
Protzel, C. et al. Lymphadenectomy in the surgical management of penile cancer. Eur. Urol. 55, 1075–1088 (2009).
Djajadiningrat, R. S. et al. Contemporary management of regional nodes in penile cancer-improvement of survival? J. Urol. 191, 68–73 (2014).
Kulkarni, J. N. & Kamat, M. R. Prophylactic bilateral groin node dissection versus prophylactic radiotherapy and surveillance in patients with N0 and N1-2A carcinoma of the penis. Eur. Urol. 26, 123–128 (1994).
Kakies, C. et al. Reproducibility of histopathologic tumor grading in penile cancer — results of a European project. Virchows Arch. 464, 453–461 (2014).
Li, Z. S. et al. Modification of N staging systems for penile cancer: a more precise prediction of prognosis. Br. J. Cancer 112, 1766–1771 (2015).
Catalona, W. J. Modified inguinal lymphadenectomy for carcinoma of the penis with preservation of saphenous veins: technique and preliminary results. J. Urol. 140, 306–310 (1988).
Spiess, P. E. et al. Preoperative lymphoscintigraphy and dynamic sentinel node biopsy for staging penile cancer: results with pathological correlation. J. Urol. 177, 2157–2161 (2007).
Slaton, J. W. et al. Tumor stage, vascular invasion and the percentage of poorly differentiated cancer: independent prognosticators for inguinal lymph node metastasis in penile squamous cancer. J. Urol. 165, 1138–1142 (2001).
Crawshaw, J. W. et al. Sentinel lymph node biopsy using dynamic lymphoscintigraphy combined with ultrasound-guided fine needle aspiration in penile carcinoma. Br. J. Radiol. 82, 41–48 (2009).
Lam, W. et al. Dynamic sentinel lymph node biopsy in patients with invasive squamous cell carcinoma of the penis: a prospective study of the long-term outcome of 500 inguinal basins assessed at a single institution. Eur. Urol. 63, 657–663 (2013).
Leijte, J. A. et al. Two-center evaluation of dynamic sentinel node biopsy for squamous cell carcinoma of the penis. J. Clin. Oncol. 27, 3325–3329 (2009).
Brouwer, O. R. et al. A hybrid radioactive and fluorescent tracer for sentinel node biopsy in penile carcinoma as a potential replacement for blue dye. Eur. Urol. 65, 600–609 (2014).
Shabbir, M., Kayes, O. & Minhas, S. Challenges and controversies in the management of penile cancer. Nat. Rev. Urol. 11, 702–711 (2014).
Sadeghi, R. et al. Accuracy of sentinel lymph node biopsy for inguinal lymph node staging of penile squamous cell carcinoma: systematic review and meta-analysis of the literature. J. Urol. 187, 25–31 (2012).
Sadeghi, R., Gholami, H., Zakavi, S. R., Kakhki, V. R. & Horenblas, S. Accuracy of 18F-FDG PET/CT for diagnosing inguinal lymph node involvement in penile squamous cell carcinoma: systematic review and meta-analysis of the literature. Clin. Nucl. Med. 37, 436–441 (2012).
Graafland, N. M. et al. Scanning with 18F-FDG-PET/CT for detection of pelvic nodal involvement in inguinal node-positive penile carcinoma. Eur. Urol. 56, 339–345 (2009).
Tabatabaei, S., Harisinghani, M. & McDougal, W. S. Regional lymph node staging using lymphotropic nanoparticle enhanced magnetic resonance imaging with ferumoxtran-10 in patients with penile cancer. J. Urol. 174, 923–927 (2005).
Pagliaro, L. C. et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J. Clin. Oncol. 28, 3851–3857 (2010).
Zargar-Shoshtari, K. et al. Establishing criteria for bilateral pelvic lymph node dissection in the management of penile cancer: lessons learned from an international multicenter collaboration. J. Urol. 194, 696–702 (2015).
Li, Z. S. et al. Disease-specific survival after radical lymphadenectomy for penile cancer: prediction by lymph node count and density. Urol. Oncol. 32, 893–900 (2014).
Zhu, Y., Zhou, X. Y., Yao, X. D., Dai, B. & Ye, D. W. The prognostic significance of p53, Ki-67, epithelial cadherin and matrix metalloproteinase-9 in penile squamous cell carcinoma treated with surgery. BJU Int. 100, 204–208 (2007).
Sharma, P. et al. MP10-11 pelvic extranodal extension leads to worse outcomes in penile cancer patients with positive pelvic lymph nodes and is associated with a survival benefit after adjuvant chemotherapy: a multi-institutional study. J. Urol. 193, e115 (2015).
Horenblas, S. et al. Detection of occult metastasis in squamous cell carcinoma of the penis using a dynamic sentinel node procedure. J. Urol. 163, 100–104 (2000).
Kroon, B. K. et al. Dynamic sentinel node biopsy in penile carcinoma: evaluation of 10 years experience. Eur. Urol. 47, 601–606 (2005).
Daseler, E. H., Anson, B. J. & Reimann, A. F. Radical excision of the inguinal and iliac lymph glands; a study based upon 450 anatomical dissections and upon supportive clinical observations. Surg. Gynecol. Obstet. 87, 679–694 (1948).
Ercole, C. E., Pow-Sang, J. M. & Spiess, P. E. Update in the surgical principles and therapeutic outcomes of inguinal lymph node dissection for penile cancer. Urol. Oncol. 31, 505–516 (2013).
Lopes, A. et al. Prognostic factors in carcinoma of the penis: multivariate analysis of 145 patients treated with amputation and lymphadenectomy. J. Urol. 156, 1637–1642 (1996).
Parra, R. O. Accurate staging of carcinoma of the penis in men with nonpalpable inguinal lymph nodes by modified inguinal lymphadenectomy. J. Urol. 155, 560–563 (1996).
Colberg, J. W., Andriole, G. L. & Catalona, W. J. Long-term follow-up of men undergoing modified inguinal lymphadenectomy for carcinoma of the penis. Br. J. Urol. 79, 54–57 (1997).
Bouchot, O., Rigaud, J., Maillet, F., Hetet, J. F. & Karam, G. Morbidity of inguinal lymphadenectomy for invasive penile carcinoma. Eur. Urol. 45, 761–765 (2004).
d'Ancona, C. A. et al. Long-term followup of penile carcinoma treated with penectomy and bilateral modified inguinal lymphadenectomy. J. Urol. 172, 498–501 (2004).
Bevan-Thomas, R., Slaton, J. W. & Pettaway, C. A. Contemporary morbidity from lymphadenectomy for penile squamous cell carcinoma: the M.D. Anderson Cancer Center Experience. J. Urol. 167, 1638–1642 (2002).
Puras-Baez, A., Rivera-Herrera, J. & Miranda, G. Role of superficial inguinal lymphadenectomy in carcinoma of the penis. J. Urol. 153, 246A (1995).
Coblentz, T. R. & Theodorescu, D. Morbidity of modified prophylactic inguinal lymphadenectomy for squamous cell carcinoma of the penis. J. Urol. 168, 1386–1389 (2002).
Lont, A. P. et al. Management of clinically node negative penile carcinoma: improved survival after the introduction of dynamic sentinel node biopsy. J. Urol. 170, 783–786 (2003).
Koifman, L., Hampl, D., Koifman, N., Vides, A. J. & Ornellas, A. A. Radical open inguinal lymphadenectomy for penile carcinoma: surgical technique, early complications and late outcomes. J. Urol. 190, 2086–2092 (2013).
Gopman, J. M. et al. Predicting postoperative complications of inguinal lymph node dissection for penile cancer in an international multicentre cohort. BJU Int. 116, 196–201 (2015).
Hinten, F. et al. Risk factors for short- and long-term complications after groin surgery in vulvar cancer. Br. J. Cancer 105, 1279–1287 (2011).
Lont, A. P. et al. Pelvic lymph node dissection for penile carcinoma: extent of inguinal lymph node involvement as an indicator for pelvic lymph node involvement and survival. J. Urol. 177, 947–952 (2007).
Horenblas, S. Lymphadenectomy in penile cancer. Urol. Clin. North Am. 38, 459–469 (2011).
Graafland, N. M., van Boven, H. H., van Werkhoven, E., Moonen, L. M. & Horenblas, S. Prognostic significance of extranodal extension in patients with pathological node positive penile carcinoma. J. Urol. 184, 1347–1353 (2010).
Johnson, D. E. & Lo, R. K. Management of regional lymph nodes in penile carcinoma. Five-year results following therapeutic groin dissections. Urology 24, 308–311 (1984).
Ravi, R. Prophylactic lymphadenectomy versus observation versus inguinal biopsy in node-negative patients with invasive carcinoma of the penis. Jpn. J. Clin. Oncol. 23, 53–58 (1993).
Ravi, R. Morbidity following groin dissection for penile carcinoma. Br. J. Urol. 72, 941–945 (1993).
Bare, R. L. et al. Inguinal lymphadenectomy and primary groin reconstruction using rectus abdominis muscle flaps in patients with penile cancer. Urology 44, 557–561 (1994).
Spratt, J. Groin dissection. J. Surg. Oncol. 73, 243–262 (2000).
Johnson, T. V. et al. Extensive inguinal lymphadenectomy improves overall 5-year survival in penile cancer patients: results from the Surveillance, Epidemiology, and End Results program. Cancer 116, 2960–2966 (2010).
Bishoff, J. et al. Endoscopic subcutaneous modified inguinal lymph node dissection (ESMIL) for squamous cell carcinoma of the penis [abstract 301]. J. Urol. 169 (Suppl.), 78 (2003).
Bishoff, J. T. in Smith's Textbook of Endourology 3rd edn Vol. 1 (eds Smith, A. D. et al.) 917–923 (Wiley-Blackwell, 2012).
Tobias-Machado, M. et al. Video endoscopic inguinal lymphadenectomy (VEIL): minimally invasive resection of inguinal lymph nodes. Int. Braz. J. Urol. 32, 316–321 (2006).
Sotelo, R. et al. Endoscopic lymphadenectomy for penile carcinoma. J. Endourol. 21, 364–367 (2007).
Master, V., Ogan, K., Kooby, D., Hsiao, W. & Delman, K. Leg endoscopic groin lymphadenectomy (LEG procedure): step-by-step approach to a straightforward technique. Eur. Urol. 56, 821–828 (2009).
Master, V. A. et al. Minimally invasive inguinal lymphadenectomy via endoscopic groin dissection: comprehensive assessment of immediate and long-term complications. J. Urol. 188, 1176–1180 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01526486 (2015).
Martin, B. M. et al. Oncologic outcomes of patients undergoing videoscopic inguinal lymphadenectomy for metastatic melanoma. J. Am. Coll. Surg. 218, 620–626 (2014).
Sommariva, A., Pasquali, S. & Rossi, C. Video endoscopic inguinal lymphadenectomy for lymph node metastasis from solid tumors. Eur. J. Surg. Oncol. 41, 274–281 (2015).
Herrel, L. A. et al. Bilateral endoscopic inguinofemoral lymphadenectomy using simultaneous carbon dioxide insufflation: an initial report of a novel approach. Can. J. Urol. 19, 6306–6309 (2012).
Matin, S. F. et al. Phase 1 prospective evaluation of the oncological adequacy of robotic assisted video-endoscopic inguinal lymphadenectomy in patients with penile carcinoma. BJU Int. 111, 1068–1074 (2013).
Kharadjian, T. B., Matin, S. F. & Pettaway, C. A. Early experience of robotic-assisted inguinal lymphadenectomy: review of surgical outcomes relative to alternative approaches. Curr. Urol. Rep. 15, 412 (2014).
Horenblas, S. et al. Squamous cell carcinoma of the penis. III. Treatment of regional lymph nodes. J. Urol. 149, 492–497 (1993).
Horenblas, S. Lymphadenectomy for squamous cell carcinoma of the penis. Part 1: diagnosis of lymph node metastasis. BJU Int. 88, 467–472 (2001).
Zhu, Y. & Ye, D.-W. Lymph node metastases and prognosis in penile cancer. Chin. J. Cancer Res. 24, 90–96 (2012).
La-Touche, S., Sangar, V., Minhas, S. & Watkin, N. 5 year cancer specific survival of patients with suspected pelvic lymph node involvement. BJU Int. 108, 6 (2011).
Solsona, E. et al. Prospective validation of the association of local tumor stage and grade as a predictive factor for occult lymph node micrometastasis in patients with penile carcinoma and clinically negative inguinal lymph nodes. J. Urol. 165, 1506–1509 (2001).
Polcari, A. J. et al. Comparison of open and robot-assisted pelvic lymphadenectomy for prostate cancer. J. Endourol. 23, 1313–1317 (2009).
Ravi, R. Correlation between the extent of nodal involvement and survival following groin dissection for carcinoma of the penis. Br. J. Urol. 72, 817–819 (1993).
Di Lorenzo, G. et al. Cisplatin and 5-fluorouracil in inoperable, stage IV squamous cell carcinoma of the penis. BJU Int. 110, E661–E666 (2012).
Theodore, C. et al. A phase II multicentre study of irinotecan (CPT 11) in combination with cisplatin (CDDP) in metastatic or locally advanced penile carcinoma (EORTC PROTOCOL 30992). Ann. Oncol. 19, 1304–1307 (2008).
Nicholson, S. et al. Phase II trial of docetaxel, cisplatin and 5FU chemotherapy in locally advanced and metastatic penis cancer (CRUK/09/001). Br. J. Cancer 109, 2554–2559 (2013).
Nicolai, N. et al. A combination of cisplatin and 5-fluorouracil with a taxane in patients who underwent lymph node dissection for nodal metastases from squamous cell carcinoma of the penis: treatment outcome and survival analyses in neoadjuvant and adjuvant settings. Clin. Genitourin. Cancer 14, 323–330 (2016).
Leijte, J. A., Kerst, J. M., Bais, E., Antonini, N. & Horenblas, S. Neoadjuvant chemotherapy in advanced penile carcinoma. Eur. Urol. 52, 488–494 (2007).
Pizzocaro, G. & Piva, L. Adjuvant and neoadjuvant vincristine, bleomycin, and methotrexate for inguinal metastases from squamous cell carcinoma of the penis. Acta Oncol. 27, 823–824 (1988).
Noronha, V. et al. Role of paclitaxel and platinum-based adjuvant chemotherapy in high-risk penile cancer. Urol. Ann. 4, 150–153 (2012).
Graafland, N. M. et al. Inguinal recurrence following therapeutic lymphadenectomy for node positive penile carcinoma: outcome and implications for management. J. Urol. 185, 888–893 (2011).
Baumgarten, A. S. et al. Salvage surgical resection for isolated locally recurrent inguinal lymph node metastasis of penile cancer: international study collaboration. J. Urol. 192, 760–764 (2014).
Block, N. L., Rosen, P. & Whitmore, W. F. Jr. Hemipelvectomy for advanced penile cancer. J. Urol. 110, 703–707 (1973).
Link, R. E., Soltes, G. D. & Coburn, M. Treatment of acute inguinal hemorrhage from metastatic penile carcinoma using an endovascular stent graft. J. Urol. 172, 1878–1879 (2004).
Blais, N. & Kassouf, E. Managing advanced penile cancer in 2014. Curr. Opin. Support. Palliat. Care 8, 241–249 (2014).
Necchi, A. et al. Proof of activity of anti-epidermal growth factor receptor-targeted therapy for relapsed squamous cell carcinoma of the penis. J. Clin. Oncol. 29, e650–e652 (2011).
Carthon, B. C., Ng, C. S., Pettaway, C. A. & Pagliaro, L. C. Epidermal growth factor receptor-targeted therapy in locally advanced or metastatic squamous cell carcinoma of the penis. BJU Int. 113, 871–877 (2014).
Haas, G. P. et al. Cisplatin, methotrexate and bleomycin for the treatment of carcinoma of the penis: a Southwest Oncology Group study. J. Urol. 161, 1823–1825 (1999).
Jensen, M. Cancer of the penis in Denmark 1942 to 1962 (511 cases). Dan. Med. Bull. 24, 66 (1977).
Horenblas, S. Lymphadenectomy for squamous cell carcinoma of the penis. Part 2: the role and technique of lymph node dissection. BJU Int. 88, 473–483 (2001).
Kunos, C., Simpkins, F., Gibbons, H., Tian, C. & Homesley, H. Radiation therapy compared with pelvic node resection for node-positive vulvar cancer: a randomized controlled trial. Obstet. Gynecol. 114, 537–546 (2009).
Pfister, D. G. et al. Head and neck cancers, version 1.2015. J. Natl Compr. Canc. Netw. 13, 847–855 (2015).
Chen, M. F. et al. Contemporary management of penile cancer including surgery and adjuvant radiotherapy: an experience in Taiwan. World J. Urol. 22, 60–66 (2004).
Ravi, R., Chaturvedi, H. K. & Sastry, D. V. Role of radiation therapy in the treatment of carcinoma of the penis. Br. J. Urol. 74, 646–651 (1994).
Burt, L. M., Shrieve, D. C. & Tward, J. D. Stage presentation, care patterns, and treatment outcomes for squamous cell carcinoma of the penis. Int. J. Radiat. Oncol. Biol. Phys. 88, 94–100 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02305654 (2014).
Buonerba, C. et al. The evolving landscape in advanced penile cancer. Future Sci. OA 1, FSO9 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02014831 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/record/NCT02104063 (2015).
Zhang, L. et al. MicroRNA expression profile in penile cancer revealed by next-generation small RNA sequencing. PLoS ONE 10, e0131336 (2015).
Vokes, E. E., Agrawal, N. & Seiwert, T. Y. HPV-associated head and neck cancer. J. Natl Cancer Inst. 107, djv344 (2015).
Miralles-Guri, C. et al. Human papillomavirus prevalence and type distribution in penile carcinoma. J. Clin. Pathol. 62, 870–878 (2009).
Lohneis, P. et al. Human papilloma virus status of penile squamous cell carcinoma is associated with differences in tumour-infiltrating T lymphocytes. Virchows Arch. 466, 323–331 (2015).
Svane, I. M. & Verdegaal, E. M. Achievements and challenges of adoptive T cell therapy with tumor-infiltrating or blood-derived lymphocytes for metastatic melanoma: what is needed to achieve standard of care? Cancer Immunol. Immunother. 63, 1081–1091 (2014).
Houot, R., Schultz, L. M., Marabelle, A. & Kohrt, H. T-Cell-based immunotherapy: adoptive cell transfer and checkpoint inhibition. Cancer Immunol. Res. 3, 1115–1122 (2015).
Wang, K., Xu, J., Zhang, T. & Xue, D. Tumor-infiltrating lymphocytes in breast cancer predict the response to chemotherapy and survival outcome: a meta-analysis. Oncotarget 7, 44288–44298 (2016).
Udager, A. M. et al. Frequent PD-L1 expression in primary and metastatic penile squamous cell carcinoma: potential opportunities for immunotherapeutic approaches. Ann. Oncol. 27, 1706–1712 (2016).
Cocks, M. et al. Immune-checkpoint status in penile squamous cell carcinoma: a North American cohort. Hum. Pathol. 59, 55–61 (2017).
Ottenhof, S. R. et al. Expression of programmed death ligand 1 in penile cancer is of prognostic value and associated with HPV status. J. Urol. 197, 690–697 (2017).
Ornellas, A. A. et al. Surgical treatment of invasive squamous cell carcinoma of the penis: retrospective analysis of 350 cases. J. Urol. 151, 1244–1249 (1994).
Author information
Authors and Affiliations
Contributions
G.J.D. and A.L. researched data for the article. G.J.D., A.L. and V.M. wrote the manuscript. All authors made substantial contributions to discussion of content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Leone, A., Diorio, G., Pettaway, C. et al. Contemporary management of patients with penile cancer and lymph node metastasis. Nat Rev Urol 14, 335–347 (2017). https://doi.org/10.1038/nrurol.2017.47
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2017.47
This article is cited by
-
Lyophilized lymph nodes for improved delivery of chimeric antigen receptor T cells
Nature Materials (2024)
-
Comparison of antegrade robotic assisted VS laparoscopic inguinal lymphadenectomy for penile cancer
BMC Surgery (2023)
-
Lymph node metastasis in cancer progression: molecular mechanisms, clinical significance and therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
Assessment and Reporting of Perioperative Adverse Events and Complications in Patients Undergoing Inguinal Lymphadenectomy for Melanoma, Vulvar Cancer, and Penile Cancer: A Systematic Review and Meta‐analysis
World Journal of Surgery (2023)
-
miR-138-5p-mediated HOXD11 promotes cell invasion and metastasis by activating the FN1/MMP2/MMP9 pathway and predicts poor prognosis in penile squamous cell carcinoma
Cell Death & Disease (2022)