Key Points
-
The identification and preservation of the cavernous nerves during radical prostatectomy is challenging, resulting in widely variable rates of postoperative sexual function
-
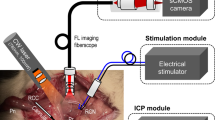
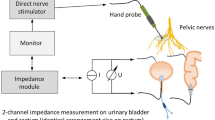
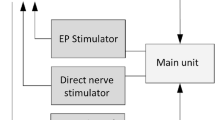
Current experimental diagnostic techniques include electrical and optical stimulation, fluorescence, microscopy, spectroscopy, ultrasonography and magnetic resonance imaging
-
These current techniques are limited by slow or inconsistent nerve responses, limited resolution, shallow imaging depth, slow image acquisition times and/or safety concerns
-
A single technology has not yet emerged as the preferred option for improving nerve-sparing radical prostatectomy
-
Multimodal approaches that combine complementary diagnostic techniques, anatomical and functional imaging and robotics might overcome some of the limitations and provide a better option
Abstract
The cavernous nerves, which course along the surface of the prostate gland, are responsible for erectile function. During radical prostatectomy, urologists are challenged in preserving these nerves and their function. Cavernous nerves are microscopic and show variable location in different patients; therefore, postoperative sexual potency rates are widely variable following radical prostatectomy. A variety of technologies, including electrical and optical nerve stimulation, dye-based optical fluorescence and microscopy, spectroscopy, ultrasound and magnetic resonance imaging have all been used to study cavernous nerve anatomy and physiology, and some of these methods are also potential intraoperative methods for identifying and preserving cavernous nerves. However, all of these technologies have inherent limitations, including slow or inconsistent nerve responses, poor image resolution, shallow image depth, slow image acquisition times and/or safety concerns. New and emerging technologies, as well as multimodal approaches combining existing methods, hold promise for improved postoperative sexual outcomes and patient quality of life following radical prostatectomy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Chen, H., Kim, C., Petrisor, D., Han, M. & Stoianovici, D. Correlation between the prostate size and location of the neurovascular bundles. J. Endourol. 27, A34–A35 (2013).
Burnett, A. L. et al. Erectile function outcome reporting after clinically localized prostate cancer treatment. J. Urol. 178, 597–601 (2007).
Costello, A. J., Brooks, M. & Cole, O. J. Anatomical studies of the neurovascular bundle and cavernosal nerves. BJU Int. 94, 1071–1076 (2004).
Kiyoshima, K. et al. Anatomical features of periprostatic tissue and its surroundings: a histological analysis of 79 radical retropubic prostatectomy specimens. Jpn J. Clin. Oncol. 34, 463–468 (2004).
Takenaka, A., Murakami, G., Matsubara, A., Han, S. H. & Fujisawa. M. Variation in course of cavernous nerve with special reference to details of topographic relationships near prostatic apex: histologic study in male cadavers. Urology 65, 136–142 (2005).
Lunacek, A., Schwentner, C., Fritsch, H., Bartsch, G. & Strasser, H. Anatomical radical retropubic prostatectomy: 'curtain dissection' of the neurovascular bundle. BJU Int. 95, 1226–1231 (2005).
Sung, W., Lee, S., Park, Y.-K. & Chang, S.-G. Neuroanatomical study of periprostatic nerve distributions using human cadaver prostate. J. Korean Med. Sci. 25, 608–612 (2010).
Alsaid, B. et al. Division of autonomic nerves within the neurovascular bundles distally into corpora cavernosa and corpus spongiosum components: immunohistochemical confirmation with three-dimensional reconstruction. Eur. Urol. 59, 902–909 (2011).
Costello, A. J., Dowdle, B. W., Namdarian, B., Pedersen, J. & Murphy, D. G. Immunohistochemical study of the cavernous nerves in the periprostatic region. BJU Int. 107, 1210–1215 (2011).
Srivastava, A. et al. Neuroanatomic basis for traction-free preservation of the neural hammock during athermal robotic radical prostatectomy. Curr. Opin. Urol. 21, 49–59 (2011).
Parekattil, S., Yeung, L. L. & Su, L. M. Intraoperative tissue characterization and imaging. Urol. Clin. North Am. 36, 213–221 (2009).
Ponnusamy, K., Sorger, J. M. & Mohr, C. Nerve mapping technologies: novel technologies under development. J. Endourol. 26, 769–777 (2012).
Rai, S., Srivastava, A., Sooriakumaran, P. & Tewari, A. Advances in imaging the neurovascular bundle. Curr. Opin. Urol. 22, 88–96 (2012).
Hsu, M., Gupta, M., Su, L. M. & Liao, J. C. Intraoperative optical imaging and tissue interrogation during urologic surgery. Curr. Opin. Urol. 24, 66–74 (2014).
Klotz, L. & Herschorn, S. Early experience with intraoperative cavernous nerve stimulation with penile tumescence monitoring to improve nerve sparing during radical prostatectomy. Urology 52, 537–542 (1998).
Klotz, L. Neurostimulation during radical prostatectomy: improving nerve-sparing techniques. Semin. Urol. Oncol. 18, 46–50 (2000).
Klotz, L. et al. A randomized phase 3 study of intraoperative cavernous nerve stimulation with penile tumescence monitoring to improve nerve sparing during radical prostatectomy. J. Urol. 164, 1573–1578 (2000).
Kim, H. L., Stoffel, D. S., Mhoon, D. A. & Brendler, C. B. A positive caver map response poorly predicts recovery of potency after radical prostatectomy. Urology 56, 561–564 (2000).
Holzbeierlein, J., Peterson, M. & Smith, J. A. Jr. Variability of results of cavernous nerve stimulation during radical prostatectomy. J. Urol. 165, 108–110 (2001).
Walsh, P. C. et al. Efficacy of first-generation Cavermap to verify location and function of cavernous nerves during radical prostatectomy: a multi-institutional study by experienced surgeons. Urology 57, 491–494 (2001).
Kim, H. L., Mhoon, D. A. & Brendler, C. B. Does the CaverMap device help preserve potency? Curr. Urol. Rep. 2, 214–217 (2001).
Kurokawa, K. et al. A simple and reliable monitoring system to confirm the preservation of the cavernous nerves. Int. J. Urol. 8, 231–236 (2001).
Kurokawa, K. et al. Preliminary results of a monitoring system to confirm the preservation of cavernous nerves. Int. J. Urol. 10, 136–140 (2003).
Takenaka, A. et al. Pelvic autonomic nerve mapping around the prostate by intraoperative electrical stimulation with simultaneous measurement of intracavernous and intraurethral pressure. J. Urol. 177, 225–229 (2007).
Tran, H. H. Development of a real time intraoperative electrical impedance tomography sensor for cavernous nerve mapping for radical prostatectomy. Can. Urol. Assoc. J. 4, S115 (2010).
Kuhn, R. Results of using a new technology to improve the identification and preservation of nerve tissue during robotic assisted laparoscopic radical prostatectomy [abstract 852]. J. Urol. 189, e350 (2013).
Kuhn, R. Real-time nerve mapping during RARP to identify nerves involved in the erectile response [abstract MP37–03]. J. Urol. 191 (Suppl.), e392–e393 (2014).
Wells. J. et al. Optical stimulation of neural tissue in vivo. Opt. Lett. 30, 504–506 (2005).
Wells, J., Kao, C., Jansen, E. D., Konrad, P. & Mahadevan-Jansen, A. Application of infrared light for in vivo neural stimulation. J. Biomed. Opt. 10, 064003, (2005).
Hale, G. M. & Querry, M. R. Optical constants of water in the 200 nm to 200 μm wavelength region. Appl. Opt. 12, 555–563 (1973).
Cesare, P., Moriondo, A., Vellani, V. & McNaughton, P. A. Ion channels gated by heat. Proc. Natl Acad. Sci. USA 96, 7658–7663 (1999).
Wells, J. et al. Biophysical mechanisms of transient optical stimulation of peripheral nerve. Biophys. J. 93, 2567–2580 (2007).
Shapiro, M. G., Homma, K., Villarreal, S., Richter, C. P. & Bezanilla, F. Infrared light excites cells by changing their electrical capacitance. Nat. Commun. 3, 736, 1–10 (2012).
Wells, J. D. et al. Optical mediated nerve stimulation: identification of injury thresholds. Lasers Surg. Med. 39, 513–526 (2007).
Fried, N. M., Lagoda, G. A., Scott, N. J., Su, L. M. & Burnett, A. L. Non-contact stimulation of the cavernous nerves in the rat prostate using a tunable-wavelength thulium fiber laser. J. Endourol. 22, 409–413 (2008).
Tozburun, S., Lagoda, G. A., Burnett, A. L. & Fried, N. M. Infrared laser nerve stimulation as a potential diagnostic method for intra-operative identification and preservation of the prostate cavernous nerves. J. Sel. Top. Quantum Electron. 20, 7101308 (2014).
Tozburun, S., Lagoda, G. A., Burnett, A. L. & Fried, N. M. Subsurface near-infrared laser stimulation of the periprostatic cavernous nerves. J. Biophotonics 5, 793–800 (2012).
Tozburun, S., Lagoda, G. A., Burnett, A. L. & Fried, N. M. Continuous-wave infrared subsurface optical stimulation of the rat prostate cavernous nerves using a 1490 nm diode laser. Urology 82, 969–973 (2013).
Tozburun, S. et al. Temperature controlled optical stimulation of the rat prostate cavernous nerves. J. Biomed. Opt. 18, 067001 (2013).
Huang, D. et al. Optical coherence tomography. Science 254, 1178–1181 (1991).
Tearney, G. J. et al. In vivo endoscopic optical biopsy with optical coherence tomography. Science 276, 2037–2039 (1997).
Koenig, F., Tearney, G. J. & Bouma, B. E. in Handbook of Optical Coherence Tomography (eds Bouma, B. E. & Tearney, G. J.) Ch. 28 (Marcel Dekker, 2002).
Crow, P., Stone, N., Kendall, C. A., Persad, R. A. & Wright, M. P. Optical diagnostics in urology: current applications and future prospects. BJU Int. 92, 400–407 (2003).
Tearney, G. J. et al. Optical biopsy in human urologic tissue using optical coherence tomography. J. Urol. 157, 1915–1919 (1997).
D'Amico, A. V., Weinstein, M., Li, X., Richie, J. P. & Fujimoto, J. Optical coherence tomography as a method for identifying benign and malignant microscopic structures in the prostate gland. Urology 55, 783–787 (2000).
Boppart, S. A. Real-time optical coherence tomography for minimally invasive imaging of prostate ablation. Comput. Aided Surg. 6, 94–103 (2001).
Aron, M. et al. Preliminary experience with the Niris optical coherence tomography system during laparoscopic and robotic prostatectomy. J. Endourol. 21, 814–818 (2007).
Fried, N. M. et al. Imaging the cavernous nerves in the rat prostate using optical coherence tomography. Lasers Surg. Med. 39, 36–41 (2007).
Rais-Bahrami, S. et al. Optical coherence tomography of cavernous nerves: a step toward real-time intraoperative imaging during nerve-sparing radical prostatectomy. Urology 72, 198–204 (2008).
Dangle, P. P. et al. The use of high resolution optical coherence tomography to evaluate robotic radical prostatectomy specimens. Int. Braz. J. Urol. 35, 344–353 (2009).
Chitchian, S., Weldon, T. P., Fiddy, M. A. & Fried, N. M. Combined image processing algorithms for improved optical coherence tomography of the prostate nerves. J. Biomed. Opt. 15, 046014, (2010).
Golijanin, D. et al. Intraoperative visualization of cavernous nerves using near-infrared fluorescence of indocyanine green in the rat. Presented at the American Urological Association Meeting, 2006.
Boyette, L. B. et al. Fiberoptic imaging of cavernous nerves in vivo. J. Urol. 178, 2694–2700 (2007).
Davila, H. H. et al. Visualization of the neurovascular bundles and major pelvic ganglion with fluorescent tracers after penile injection in the rat. BJU Int. 101, 1048–1051 (2008).
Tuttle, J. B. & Steers, W. D. Fiberoptic imaging for urologic surgery. Curr. Urol. Rep. 10, 60–64 (2009).
Lee, K. C., Sharma, S., Tuttle, J. B. & Steers, W. D. Origin and characterization of retrograde labeled neurons supplying the rat urethra using fiberoptic confocal fluorescent microscopy in vivo and immunohistochemistry. J. Urol. 184, 1550–1554 (2010).
Chaux, A. et al. Focal positive prostate-specific membrane antigen (PSMA) expression in ganglionic tissues associated with prostate neurovascular bundle: implications for novel intraoperative PSMA-based fluorescent imaging techniques. Urol. Oncol. 31, 572–575 (2013).
Zaak, D. et al. Photodynamic diagnosis of prostate cancer using 5-aminolevulinic acid—first clinical experiences. Urology 72, 345–348 (2008).
Ganzer, R. et al. Intraoperative photodynamic evaluation of surgical margins during endoscopic extraperitoneal radical prostatectomy with the use of 5-aminolevulinic acid. J. Endourol. 23, 1387–1394 (2009).
Fukuhara, H. et al. Photodynamic diagnosis of positive surgical margin during radical prostatectomy: preliminary experience with 5-aminolevulinic acid. Int. J. Urol. 18, 585–591 (2011).
Inoue, K. et al. Application of 5-aminolevulinic acid-mediated photodynamic diagnosis to robot-assisted laparoscopic radical prostatectomy. Urology 82, 1175–1178 (2013).
KleinJan, G. H. et al. Optimisation of fluorescence guidance during robot-assisted laparoscopic sentinel node biopsy for prostate cancer. Eur. Urol. 66, 991–998 (2014).
Wu, K. et al. Dynamic real-time microscopy of the urinary tract using confocal laser endomicroscopy. Urology 78, 225–231 (2011).
Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Yadav, R. et al. Multiphoton microscopy of prostate and periprostatic neural tissue: a promising technique for improving nerve-sparing prostatectomy. J. Endourol. 23, 861–867 (2009).
Grover, S. G. et al. Real-time multiphoton microscopy of human periprostatic tissue architecture for improving identification of vital tissue structures during nerve-sparing radical prostatectomy. Br. J. Med. Surg. Urol. 3, 267–268 (2010).
Tewari, A. K. et al. Multiphoton microscopy for structure identification in human prostate and periprostatic tissue: implications in prostate cancer surgery. BJU Int. 108, 1421–1429 (2011).
Durand, M. et al. Real-time in vivo periprostatic nerve tracking using multiphoton microscopy in a rat survival surgery model: a promising pre-clinical study for enhanced nerve-sparing surgery. BJU Int. http://dx.doi.org/10.1111/bju.12903.
Gao, L. et al. Label-free high-resolution imaging of prostate glands and cavernous nerves using coherent anti-Stokes Raman scattering microscopy. Biomed. Opt. Express 2, 915–926 (2011).
Crow, P. et al. Assessment of fiber optic near-infrared Raman spectroscopy for diagnosis of bladder and prostate cancer. Urology 65, 1126–1130 (2005).
A'Amar, O. M., Liou, L., Rodriguez-Diaz, E., De las Morenas, A. & Bijio, I. J. Comparison of elastic scattering spectroscopy with histology in ex vivo prostate glands: potential application for optically guided biopsy and directed treatment. Lasers Med. Sci. 28, 1323–1329 (2013).
Baykara, M. et al. Detecting positive surgical margins using single optical fiber probe during radical prostatectomy: a pilot study. Urology 83, 1438–1442 (2014).
Ukimura, O. et al. Real-time ultrasonography during laparoscopic radical prostatectomy. J. Urol. 172, 112–118, (2004).
Ukimura, O. & Gill, I. S. Real-time transrectal ultrasound guidance during nerve sparing laparoscopic radical prostatectomy: pictorial essay. J. Urol. 175, 1311–1319 (2006).
Han, M. et al. Tandem-robot assisted laparoscopic radical prostatectomy to improve the neurovascular bundle visualization: a feasibility study. Urology 77, 502–506 (2011).
Long, J. A. et al. Real-time robotic transrectal ultrasound navigation during robotic radical prostatectomy: initial clinical experience. Urology 80, 608–613 (2012).
Badani, K. K. et al. A pilot study of laparoscopic Doppler ultrasound probe to map arterial vascular flow within the neurovascular bundle during robot-assisted radical prostatectomy. Prostate Cancer http://dx.doi.org/10.1155/2013/810715.
Axelson, H. W., Johansson, E. & Bill-Axelson, A. Intraoperative cavernous nerve stimulation and laser-doppler flowmetry during radical prostatectomy. J. Sex. Med. 10, 2842–2848 (2013).
Tsai, Y. S. et al. Doppler spectral waveform parameters at neurovascular bundle vessels in patients with prostate biopsy. J. Endourol. 28, 364–370 (2014).
Pavlovich, C. P. et al. High-resolution transrectal ultrasound: pilot study of a novel technique for imaging clinically localized prostate cancer. Urol. Oncol. 32, 34e27–34e32 (2014).
Lee, S. H. et al. Significance of neurovascular bundle formation observed on preoperative magnetic resonance imaging regarding postoperative erectile function after nerve-sparing retropubic radical prostatectomy. Urology 69, 510–514 (2007).
Brown, J. A. et al. Impact of preoperative endorectal MRI stage classification on neurovascular bundle sparing aggressiveness and the radical prostatectomy positive margin rate. Urol. Oncol. 27, 174–179 (2009).
Labanaris, A. P. et al. The role of conventional and functional endorectal magnetic resonance imaging in the decision of whether to preserve or resect the neurovascular bundles during radical retropubic prostatectomy. Scand. J. Urol. Nephrol. 43, 25–31 (2009).
Panebianco, V. et al. Use of multiparametric MR with neurovascular bundle evaluation to optimize the oncological and functional management of patients considered for nerve-sparing radical prostatectomy. J. Sex. Med. 9, 2157–2166 (2012).
McClure, T. D. et al. Use of MR imaging to determine preservation of the neurovascular bundles at robotic-assisted laparoscopic prostatectomy. Radiology 262, 874–883 (2012).
Tanaka, K. et al. Efficacy of using three-tesla magnetic resonance imaging diagnosis of capsule invasion for decision-making about neurovascular bundle preservation in robotic-assisted radical prostatectomy. Korean J. Urol. 54, 437–441 (2013).
Panebianco, V. et al. In vivo 3D neuroanatomical evaluation of periprostatic nerve plexus with 3T-MR Diffusion Tensor Imaging. Eur. J. Radiol. 82, 1677–1682 (2013).
Park, B. H. et al. Influence of magnetic resonance imaging in the decision to preserve or resect neurovascular bundles at robotic assisted laparoscopic radical prostatectomy. J. Urol. 192, 82–88 (2014).
Hoeks, C. M. et al. Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology 261, 46–66 (2011).
Hegde, J. V. et al. Multiparametric MRI of prostate cancer; an update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. J. Magn. Reson. Imaging 37, 1035–1054 (2013).
Mass, M. C. et al. Feasibility of T2-weighted turbo spin echo imaging of the human prostate gland at 7 tesla. Magn. Reson. Med. 71, 1711–1719 (2014).
Vos, E. K. et al. Image quality and cancer visibility of T2-weighted magnetic resonance imaging of the prostate at 7 tesla. Eur. Radiol. 24, 1950–1958 (2014).
Luttje, M. P. et al. (31) P MR spectroscopic imaging combined with (1) H MR spectroscopic imaging in the human prostate using a double tuned endorectal coil at 7T. Magn. Reson. Med. 72, 1516–1521 (2014).
Rosenkrantz, A. B. et al. T2-weighted prostate MRI at 7 Tesla using a simplified external transmit-receive coil array: correlation with radical prostatectomy findings in two prostate cancer patients. J. Magn. Reson. Imaging 41, 226–232 (2015).
Wang, X., Roberts, W. W., Carson, P. L., Wood, D. P. & Fowlkes, J. B. Photoacoustic tomography: a potential new tool for prostate cancer. Biomed. Opt. Express 7, 1117–1126 (2010).
Wang, L. V. & Hu, S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 335, 1458–1462 (2012).
Duke, A. R. et al. Combined optical and electrical stimulation of neural tissue in vivo. J. Biomed. Opt. 14, 060501 (2009).
Duke, A. R., Lu, H., Jenkins, M. W., Chiel, H. J. & Jansen, E. D. Spatial and temporal variability in response to hybrid electro-optical stimulation. J. Neural Eng. 9, 036003, 1–15 (2012).
Duke, A. R. et al. Hybrid electro-optical stimulation of the rat sciatic nerve induces force generation in the plantarflexor muscles. J. Neural Eng. 9, 066006, 1–12 (2012).
Duke, A. R., Chiel, H. J. & Jansen, E. D. A simple model of the combined electrical-optical interaction in neural tissue. Austin J. Biomed. Eng. 1, 1010 (2014).
Tozburun, S. et al. A compact laparoscopic probe for optical stimulation of the prostate nerves. J. Sel. Top. Quantum Electron. 16, 941–945 (2010).
Patil, A. V., Garson, C. D. & Hossack, J. A. 3D prostate elastography; algorithm, simulations and experiments. Phys. Med. Biol. 52, 3643–3663 (2007).
Fleming, I. N. et al. Ultrasound elastography: enabling technology for image guided laparoscopic prostatectomy. Proc. SPIE 7261, 72612I (2009).
Fleming, I. N. et al. Ultrasound elastography as a tool for imaging guidance during prostatectomy: initial experience. Med. Sci. Monit. 18, CR635–CR642 (2012).
Author information
Authors and Affiliations
Contributions
N.M.F. researched data for the article and wrote the manuscript. A.L.B. contributed to the discussion of content and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Fried, N., Burnett, A. Novel methods for mapping the cavernous nerves during radical prostatectomy. Nat Rev Urol 12, 451–460 (2015). https://doi.org/10.1038/nrurol.2015.174
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2015.174
This article is cited by
-
Infrared neural stimulation markedly enhances nerve functionality assessment during nerve monitoring
Scientific Reports (2023)
-
Real-time, functional intra-operative localization of rat cavernous nerve network using near-infrared cyanine voltage-sensitive dye imaging
Scientific Reports (2020)
-
Stimulators and activators of soluble guanylate cyclase for urogenital disorders
Nature Reviews Urology (2018)