Abstract
Optimal fracture treatment requires knowledge of the complex physiological process of bone healing. The course of bone healing is mainly influenced by fracture fixation stability (biomechanics) and the blood supply to the healing site (revascularization after trauma). The repair process proceeds via a characteristic sequence of events, described as the inflammatory, repair and remodeling phases. An inflammatory reaction involving immune cells and molecular factors is activated immediately in response to tissue damage and is thought to initiate the repair cascade. Immune cells also have a major role in the repair phase, exhibiting important crosstalk with bone cells. After bony bridging of the fragments, a slow remodeling process eventually leads to the reconstitution of the original bone structure. Systemic inflammation, as observed in patients with rheumatoid arthritis, diabetes mellitus, multiple trauma or sepsis, can increase fracture healing time and the rate of complications, including non-unions. In addition, evidence suggests that insufficient biomechanical conditions within the fracture zone can influence early local inflammation and impair bone healing. In this Review, we discuss the main factors that influence fracture healing, with particular emphasis on the role of inflammation.
Key Points
-
Fracture healing is a complex, highly regulated process with consecutive and closely linked phases of inflammation, repair and remodeling
-
Optimal fracture healing requires suitable biological as well as biomechanical conditions
-
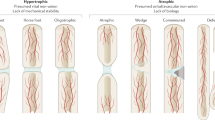
The mechanical environment considerably influences tissue differentiation during bone healing: stable fracture fixation induces direct bone formation, moderate stability provokes endochondral ossification, whereas unstable fixation inhibits bone healing
-
The immune system is intimately involved in the fracture healing process, especially during the early inflammatory healing phase
-
Disorders associated with systemic inflammation, such as diabetes mellitus, trauma, sepsis and rheumatoid arthritis, can prolong or disturb fracture healing and increase the risk of non-unions by incompletely understood mechanisms
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Buckwalter, J. A., Einhorn, T. A., Bolander, M. E. & Cruess, R. L. in Rockwood and Green's Fractures in Adults, 4th edn (eds Rockwood, C. A. Jr. et al.) 261–304 (Lippincott-Raven, Philadelphia, 1996).
Sarmiento, A. & Latta, L. L. Closed Functional Treatment of Fractures (Springer-Verlag, New York; Berlin, 1981).
Rüedi, T. P. & Murphy, W. M. (eds) AO Principles of Fracture Management (Thieme, Stuttgart, 2000).
Bhandari, M. et al. Predictors of reoperation following operative management of fractures of the tibial shaft. J. Orthop. Trauma 17, 353–361 (2003).
Karladani, A. H., Granhed, H., Kärrholm, J. & Styf, J. The influence of fracture etiology and type on fracture healing: a review of 104 consecutive tibial shaft fractures. Arch. Orthop. Trauma Surg. 121, 325–328 (2001).
Hayda, R. A., Brighton, C. T. & Esterhai, J. L. Jr. Pathophysiology of delayed healing. Clin. Orthop. Relat. Res. 355 (Suppl.), S31–S40 (1998).
McKibbin, B. The biology of fracture healing in long bones. J. Bone Joint Surg. Br. 60-B, 150–162 (1978).
Claes, L. E. & Cunningham, J. L. Monitoring the mechanical properties of healing bone. Clin. Orthop. Relat. Res. 467, 1964–1971 (2009).
Histing, T. et al. Ex vivo analysis of rotational stiffness of different osteosynthesis techniques in mouse femur fracture. J. Orthop. Res. 27, 1152–1156 (2009).
Histing, T. et al. Small animal bone healing models: standards, tips, and pitfalls results of a consensus meeting. Bone 49, 591–599 (2011).
Willie, B., Adkins, K., Zheng, X., Simon, U. & Claes, L. Mechanical characterization of external fixator stiffness for a rat femoral fracture model. J. Orthop. Res. 27, 687–693 (2009).
Einhorn, T. A. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res., 355 (Suppl.), S7–S21 (1998).
Gerstenfeld, L. C., Cullinane, D. M., Barnes, G. L., Graves, D. T. & Einhorn, T. A. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J. Cell. Biochem. 88, 873–884 (2003).
Perren, S. M. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J. Bone Joint Surg. Br. 84-B, 1093–1110 (2002).
Claes, L. Biomechanical principles and mechanobiologic aspects of flexible and locked plating. J. Orthop. Trauma 25 (Suppl. 1), S4–S7 (2011).
Claes, L. E. & Heigele, C. A. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J. Biomech. 32, 255–266 (1999).
Claes, L. E. & Ito, K. in Basic Orthopaedic Biomechanics and Mechano-Biology, 3rd edn (eds Mow, V. C. & Huiskes, R.) 563–584 (Lippincott Williams & Wilkins, Philadelphia, 2005).
Duda, G. N. et al. Mechanical behavior of Ilizarov ring fixators. Effect of frame parameters on stiffness and consequences for clinical use. Unfallchirurg 103, 839–845 (2000).
Duda, G. N. et al. Interfragmentary motion in tibial osteotomies stabilized with ring fixators. Clin. Orthop.Relat. Res. 396, 163–172 (2002).
Augat, P. et al. Shear movement at the fracture site delays healing in a diaphyseal fracture model. J. Orthop. Res. 21, 1011–1017 (2003).
Augat, P. et al. Early, full weightbearing with flexible fixation delays fracture healing. Clin. Orthop. Relat. Res. 328, 194–202 (1996).
Claes, L., Augat, P., Suger, G. & Wilke, H. J. Influence of size and stability of the osteotomy gap on the success of fracture healing. J. Orthop. Res. 15, 577–584 (1997).
Epari, D. R., Schell, H., Bail, H. J. & Duda, G. N. Instability prolongs the chondral phase during bone healing in sheep. Bone 38, 864–870 (2006).
Küntscher, G. Praxis der Marknagelung [German] (Schattauer, Stuttgart, 1962).
Duda, G. N. et al. Mechanical boundary conditions of fracture healing: borderline indications in the treatment of unreamed tibial nailing. J. Biomech. 34, 639–650 (2001).
Schandelmaier, P., Krettek, C. & Tscherne, H. Biomechanical study of nine different tibia locking nails. J. Orthop. Trauma 10, 37–44 (1996).
Wehner, T., Penzkofer, R., Augat, P., Claes, L. & Simon, U. Improvement of the shear fixation stability of intramedullary nailing. Clin. Biomech. (Bristol, Avon) 26, 147–151 (2011).
Bottlang, M. et al. Far cortical locking can improve healing of fractures stabilized with locking plates. J. Bone Joint Surg. Am. 92, 1652–1660 (2010).
Uhthoff, H. K., Goto, S. & Cerckel, P. H. Influence of stable fixation on trabecular bone healing: a morphologic assessment in dogs. J. Orthop. Res. 5, 14–22 (1987).
Claes, L. et al. Moderate soft tissue trauma delays new bone formation only in the early phase of fracture healing. J. Orthop. Res. 24, 1178–1185 (2006).
Grundnes, O. & Reikeras, O. Blood flow and mechanical properties of healing bone. Femoral osteotomies studied in rats. Acta Orthop. Scand. 63, 487–491 (1992).
Melnyk, M., Henke, T., Claes, L. & Augat, P. Revascularisation during fracture healing with soft tissue injury. Arch. Orthop. Trauma Surg. 128, 1159–1165 (2008).
Mohanti, R. C. & Mahakul, N. C. Vascular response in fractured limbs with and without immobilisation: an experimental study on rabbits. Int. Orthop. 7, 173–177 (1983).
Brookes, M. & Revell, W. J. Blood Supply of Bone: Scientific Aspects (Springer, London, 1998).
Rhinelander, F. W. Tibial blood supply in relation to fracture healing. Clin. Orthop. Relat. Res. 105, 34–81 (1974).
Strachan, R. K., McCarthy, I., Fleming, R. & Hughes, S. P. The role of the tibial nutrient artery. Microsphere estimation of blood flow in the osteotomised canine tibia. J. Bone Joint Surg. Br. 72-B, 391–394 (1990).
Triffitt, P. D., Cieslak, C. A. & Gregg, P. J. A quantitative study of the routes of blood flow to the tibial diaphysis after an osteotomy. J. Orthop. Res. 11, 49–57 (1993).
Claes, L., Heitemeyer, U., Krischak, G., Braun, H. & Hierholzer, G. Fixation technique influences osteogenesis of comminuted fractures. Clin. Orthop. Relat. Res. 365, 221–229 (1999).
Smith, S. R., Bronk, J. T. & Kelly, P. J. Effect of fracture fixation on cortical bone blood flow. J. Orthop. Res. 8, 471–478 (1990).
Olerud, S. & Strömberg, L. Intramedullary reaming and nailing: its early effects on cortical bone vascularization. Orthopedics 9, 1204–1208 (1986).
Wallace, A. L., Draper, E. R., Strachan, R. K., McCarthy, I. D. & Hughes, S. P. The vascular response to fracture micromovement. Clin. Orthop. Relat. Res. 301, 281–290 (1994).
Claes, L., Eckert-Hübner, K. & Augat, P. The effect of mechanical stability on local vascularization and tissue differentiation in callus healing. J. Orthop. Res. 20, 1099–1105 (2002).
Wallace, A. L., Draper, E. R., Strachan, R. K., McCarthy, I. D. & Hughes, S. P. The effect of devascularisation upon early bone healing in dynamic external fixation. J. Bone Joint Surg. Br. 73-B, 819–825 (1991).
Utvåg, S. E., Grundnes, O., Rindal, D. B. & Reikerås, O. Influence of extensive muscle injury on fracture healing in rat tibia. J. Orthop. Trauma 17, 430–435 (2003).
Claes, L. et al. The effect of both a thoracic trauma and a soft-tissue trauma on fracture healing in a rat model. Acta Orthop. 82, 223–227 (2011).
Hausman, M. R., Schaffler, M. B. & Majeska, R. J. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone 29, 560–564 (2001).
Kolar, P. et al. The early fracture hematoma and its potential role in fracture healing. Tissue Eng. Part B Rev. 16, 427–434 (2010).
Wray, J. B. Acute changes in femoral arterial blood flow after closed tibial fracture in dogs. J. Bone Joint Surg. Am. 46, 1262–1268 (1964).
Aho, A. J. Electron microscopic and histological observations on fracture repair in young and old rats. Acta Pathol. Microbiol. Scand. 184 (Suppl.), 1–95 (1966).
Kolar, P., Gaber, T., Perka, C., Duda, G. N. & Buttgereit, F. Human early fracture hematoma is characterized by inflammation and hypoxia. Clin. Orthop. Relat. Res. 469, 3118–3126 (2011).
Chung, R., Cool, J. C., Scherer, M. A., Foster, B. K. & Xian, C. J. Roles of neutrophil-mediated inflammatory response in the bony repair of injured growth plate cartilage in young rats. J. Leukoc. Biol. 80, 1272–1280 (2006).
Bastian, O. et al. Systemic inflammation and fracture healing. J. Leukoc. Biol. 89, 669–673 (2011).
Andrew, J. G., Andrew, S. M., Freemont, A. J. & Marsh, D. R. Inflammatory cells in normal human fracture healing. Acta Orthop. Scand. 65, 462–466 (1994).
Xing, Z. et al. Multiple roles for CCR2 during fracture healing. Dis. Model. Mech. 3, 451–458 (2010).
Gökturk, E. et al. Oxygen-free radicals impair fracture healing in rats. Acta Orthop. Scand. 66, 473–475 (1995).
Alexander, K. et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J. Bone Miner. Res. 26, 1517–1532 (2011).
Ai-Aql, Z. S., Alagl, A. S., Graves, D. T., Gerstenfeld, L. C. & Einhorn, T. A. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J. Dent. Res. 87, 107–118 (2008).
Kon, T. et al. Expression of osteoprotegerin, receptor activator of NF-κB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J. Bone Miner. Res. 16, 1004–1014 (2001).
Schmidt-Bleek, K. et al. Inflammatory phase of bone healing initiates the regenerative healing cascade. Cell Tissue Res. http://dx.doi.org/10.1007/s00441-011-1205-7.
Lienau, J. et al. Differential regulation of blood vessel formation between standard and delayed bone healing. J. Orthop. Res. 27, 1133–1140 (2009).
Decker, B., Bartels, H. & Decker, S. Relationships between endothelial cells, pericytes, and osteoblasts during bone formation in the sheep femur following implantation of tricalciumphosphate-ceramic. Anat. Rec. 242, 310–320 (1995).
Marsell, R. & Einhorn, T. A. The biology of fracture healing. Injury 42, 551–555 (2011).
Einhorn, T. A. The science of fracture healing. J. Orthop. Trauma 19, S4–S6 (2005).
Cho, T. J., Gerstenfeld, L. C. & Einhorn, T. A. Differential temporal expression of members of the transforming growth factor β superfamily during murine fracture healing. J. Bone Miner. Res. 17, 513–520 (2002).
Granero-Moltó, F. et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 27, 1887–1898 (2009).
Cottrell, J. & O'Connor, J. P. Effect of non-steroidal anti-inflammatory drugs on bone healing. Pharmaceuticals 3, 1668–1693 (2010).
Naik, A. A. et al. Reduced COX-2 expression in aged mice is associated with impaired fracture healing. J. Bone Miner. Res. 24, 251–264 (2009).
Harder, A. T. & An, Y. H. The mechanisms of the inhibitory effects of nonsteroidal anti-inflammatory drugs on bone healing: a concise review. J. Clin. Pharmacol. 43, 807–815 (2003).
Perren, S. M. & Claes, L. in Fracture Management in AO Principles of Fracture Management (eds Rüedi, T. P. & Murphy, W. M.) 7–30 (Thieme-Verlag, Stuttgart-New York, 2000).
Willenegger, H., Perren, S. M. & Schenk, R. Primary and secondary healing of bone fractures. Chirurg. 42, 241–252 (1971).
Claes, L. et al. Monitoring and healing analysis of 100 tibial shaft fractures. Langenbecks Arch. Surg. 387, 146–152 (2002).
Duda, G. N. et al. Mechanical conditions in the internal stabilization of proximal tibial defects. Clin. Biomech. (Bristol, Avon) 17, 64–72 (2002).
Nakahara, H. et al. Bone and cartilage formation in diffusion chambers by subcultured cells derived from the periosteum. Bone 11, 181–188 (1990).
Bassett, C. A. & Herrmann, I. Influence of oxygen concentration and mechanical factors on differentiation of connective tissues in vitro. Nature 190, 460–461 (1961).
Phillips, A. M. Overview of the fracture healing cascade. Injury 36 (Suppl. 3), S5–S7 (2005).
Claes, L. et al. Metaphyseal fracture healing follows similar biomechanical rules as diaphyseal healing. J. Orthop. Res. 29, 425–432 (2011).
Jarry, L. & Uhthoff, H. K. Differences in healing of metaphyseal and diaphyseal fractures. Can. J. Surg. 14, 127–135 (1971).
Uhthoff, H. K. & Rahn, B. A. Healing patterns of metaphyseal fractures. Clin. Orthop. Relat. Res. 160, 295–303 (1981).
Schatzker, J., Waddell, J. & Stoll, J. E. The effects of motion on the healing of cancellous bone. Clin. Orthop. Relat. Res. 245, 282–287 (1989).
Aronson, J. & Shen, X. Experimental healing of distraction osteogenesis comparing metaphyseal with diaphyseal sites. Clin. Orthop. Relat. Res. 301, 25–30 (1994).
Fan, W., Crawford, R. & Xiao, Y. Structural and cellular differences between metaphyseal and diaphyseal periosteum in different aged rats. Bone 42, 81–89 (2008).
Hardy, R. & Cooper, M. S. Bone loss in inflammatory disorders. J. Endocrinol. 201, 309–320 (2009).
Clowes, J. A., Riggs, B. L. & Khosla, S. The role of the immune system in the pathophysiology of osteoporosis. Immunol. Rev. 208, 207–227 (2005).
Alexandraki, K. I. et al. Cytokine secretion in long-standing diabetes mellitus type 1 and 2: associations with low-grade systemic inflammation. J. Clin. Immunol. 28, 314–321 (2008).
O'Rourke, R. W. Molecular mechanisms of obesity and diabetes: at the intersection of weight regulation, inflammation, and glucose homeostasis. World J. Surg. 33, 2007–2013 (2009).
Cao, J. J. Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 6, 30 (2011).
Loder, R. T. The influence of diabetes mellitus on the healing of closed fractures. Clin. Orthop. Relat. Res. 232, 210–216 (1988).
Alblowi, J. et al. High levels of tumor necrosis factor-α contribute to accelerated loss of cartilage in diabetic fracture healing. Am. J. Pathol. 175, 1574–1585 (2009).
Kayal, R. A. et al. TNF-α mediates diabetes-enhanced chondrocyte apoptosis during fracture healing and stimulates chondrocyte apoptosis through FOXO1. J. Bone Miner. Res. 25, 1604–1615 (2010).
Kayal, R. A. et al. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J. Bone Miner. Res. 22, 560–568 (2007).
Strömqvist, B. Hip fracture in rheumatoid arthritis. Acta Orthop. Scand. 55, 624–628 (1984).
Dominiak, B., Oxberry, W. & Chen, P. Study on a nonhealing fracture from a patient with systemic lupus erythematosus and its pathogenetic mechanisms. Ultrastruct. Pathol. 29, 107–120 (2005).
Grøgaard, B., Gerdin, B. & Reikerås, O. The polymorphonuclear leukocyte: has it a role in fracture healing? Arch. Orthop. Trauma Surg. 109, 268–271 (1990).
Keel, M. & Trentz, O. Pathophysiology of polytrauma. Injury 36, 691–709 (2005).
Recknagel, S. et al. Experimental blunt chest trauma impairs fracture healing in rats. J. Orthop. Res. 29, 734–739 (2011).
Recknagel, S. et al. C5aR-antagonist significantly reduces the deleterious effect of a blunt chest trauma on fracture healing. J. Orthop. Res. http://dx.doi.org/10.1002/jor.21561.
Reikerås, O., Shegarfi, H., Wang, J. E. & Utvåg, S. E. Lipopolysaccharide impairs fracture healing: an experimental study in rats. Acta Orthop. 76, 749–753 (2005).
Champagne, C. M., Takebe, J., Offenbacher, S. & Cooper, L. F. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone 30, 26–31 (2002).
Grundnes, O. & Reikeraas, O. Effects of macrophage activation on bone healing. J. Orthop. Sci. 5, 243–247 (2000).
Gerstenfeld, L. C. et al. Impaired fracture healing in the absence of TNF-α signaling: the role of TNF-α in endochondral cartilage resorption. J. Bone Miner. Res. 18, 1584–1592 (2003).
Yang, X. et al. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone 41, 928–936 (2007).
Wallace, A., Cooney, T. E., Englund, R. & Lubahn, J. D. Effects of interleukin-6 ablation on fracture healing in mice. J. Orthop. Res. 29, 1437–1442 (2011).
Bogoch, E., Gschwend, N., Rahn, B., Moran, E. & Perren, S. Healing of cancellous bone osteotomy in rabbits—Part I: regulation of bone volume and the regional acceleratory phenomenon in normal bone. J. Orthop. Res. 11, 285–291 (1993).
Bogoch, E., Gschwend, N., Rahn, B., Moran, E. & Perren, S. Healing of cancellous bone osteotomy in rabbits—Part II: local reversal of arthritis-induced osteopenia after osteotomy. J. Orthop. Res. 11, 292–298 (1993).
Wang, H. et al. Thrombin peptide (TP508) promotes fracture repair by up-regulating inflammatory mediators, early growth factors, and increasing angiogenesis. J. Orthop. Res. 23, 671–679 (2005).
Hankemeier, S. et al. Alteration of fracture stability influences chondrogenesis, osteogenesis and immigration of macrophages. J. Orthop. Res. 19, 531–538 (2001).
Toben, D. et al. Fracture healing is accelerated in the absence of the adaptive immune system. J. Bone Miner. Res. 26, 113–124 (2011).
Giannoudis, P. V., Einhorn, T. A. & Marsh, D. Fracture healing: the diamond concept. Injury 38 (Suppl. 4), S3–S6 (2007).
Gaston, M. S. & Simpson, A. H. Inhibition of fracture healing. J. Bone Joint Surg. Br. 89-B, 1553–1560 (2007).
Dimitriou, R., Jones, E., McGonagle, D. & Giannoudis, P. V. Bone regeneration: current concepts and future directions. BMC Med. 9, 66 (2011).
Komatsu, D. E. & Warden, S. J. The control of fracture healing and its therapeutic targeting: improving upon nature. J. Cell. Biochem. 109, 302–311 (2010).
Tosounidis, T., Kontakis, G., Nikolaou, V., Papathanassopoulos, A. & Giannoudis, P. V. Fracture healing and bone repair: an update. Trauma 11, 145–156 (2009).
Keramaris, N. C., Calori, G. M., Nikolaou, V. S., Schemitsch, E. H. & Giannoudis, P. V. Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury 39 (Suppl. 2), S45–S57 (2008).
Graham, S. et al. Investigating the role of PDGF as a potential drug therapy in bone formation and fracture healing. Expert Opin. Investig. Drugs 18, 1633–1654 (2009).
Schmidmaier, G. et al. Improvement of fracture healing by systemic administration of growth hormone and local application of insulin-like growth factor-1 and transforming growth factor-β1. Bone 31, 165–172 (2002).
Nielsen, H. M., Bak, B., Jørgensen, P. H. & Andreassen, T. T. Growth hormone promotes healing of tibial fractures in the rat. Acta Orthop. Scand. 62, 244–247 (1991).
Kolbeck, S. et al. Homologous growth hormone accelerates bone healing--a biomechanical and histological study. Bone 33, 628–637 (2003).
Epari, D. R., Kassi, J. P., Schell, H. & Duda, G. N. Timely fracture-healing requires optimization of axial fixation stability. J. Bone Joint Surg. Am. 89, 1575–1585 (2007).
Kaspar, K. et al. Angle stable locking reduces interfragmentary movements and promotes healing after unreamed nailing. Study of a displaced osteotomy model in sheep tibiae. J. Bone Joint Surg. Am. 87, 2028–2037 (2005).
Röntgen, V. et al. Fracture healing in mice under controlled rigid and flexible conditions using an adjustable external fixator. J. Orthop. Res. 28, 1456–1462 (2010).
Bashardoust Tajali, S., Houghton, P., Macdermid, J. C. & Grewal, R. Effects of low-intensity pulsed ultrasound therapy on fracture healing: a systematic review and meta-analysis. Am. J. Phys. Med. Rehabil. http://dx.doi.org/10.1097/PHM.0b013e31822419ba.
Li, X. et al. Dickkopf-1 regulates bone formation in young growing rodents and upon traumatic injury. J. Bone Miner. Res. 26, 2610–2621 (2011).
Li, C. et al. Increased callus mass and enhanced strength during fracture healing in mice lacking the sclerostin gene. Bone 49, 1178–1185 (2011).
Xie, C. et al. Rescue of impaired fracture healing in Cox-2−/− mice via activation of prostaglandin E2 receptor subtype 4. Am. J. Pathol. 175, 772–785 (2009).
Delgado-Martínez, A. D., Martínez, M. E., Carrascal, M. T., Rodríguez-Avial, M. & Munuera, L. Effect of 25-OH-vitamin D on fracture healing in elderly rats. J. Orthop. Res. 16, 650–653 (1998).
Turk, C. et al. Promotion of fracture healing by vitamin E in rats. J. Int. Med. Res. 32, 507–512 (2004).
Sarisözen, B., Durak, K., Dinçer, G. & Bilgen, O. F. The effects of vitamins E and C on fracture healing in rats. J. Int. Med. Res. 30, 309–313 (2002).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all stages of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Claes, L., Recknagel, S. & Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol 8, 133–143 (2012). https://doi.org/10.1038/nrrheum.2012.1
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2012.1
This article is cited by
-
Modulation of fracture healing by senescence-associated secretory phenotype (SASP): a narrative review of the current literature
European Journal of Medical Research (2024)
-
Temporal gene expression profiling during early-stage traumatic temporomandibular joint bony ankylosis in a sheep model
BMC Oral Health (2024)
-
Segmental femoral fracture malunion: evidence and prognostic analysis of medical intervention in the third century BC
Scientific Reports (2024)
-
Magnesium promotes vascularization and osseointegration in diabetic states
International Journal of Oral Science (2024)
-
Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: a narrative review
Cellular & Molecular Biology Letters (2023)