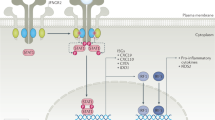
Abstract
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease involving most immune cells. Studies in both experimental animal models of lupus and patients with SLE have revealed a number of cytokine pathways that are important in the disease process. Among these are B-cell activating factor, which promotes B-cell survival and autoantibody production, interferon-α, which acts as an immune adjuvant, and tumor necrosis factor, which contributes to organ inflammation. This knowledge, in combination with the successful use of anti-TNF treatment in rheumatoid arthritis, has spurred the development of several biologic agents targeting different cytokines or their receptors in SLE. Consequently, many trials of anticytokine therapies for SLE are underway. Although most of these trials are small or in early phases, the results of some large studies have also been reported. In this Review, we discuss the rationale for anticytokine therapies in SLE and review agents currently in use, and those being developed and tested experimentally. We present the results from published trials and discuss the tentative conclusions that can be drawn regarding the efficacy of the new agents. Finally, we provide suggestions for the future of treatment for SLE, including new therapeutic strategies.
Key Points
-
Many cytokine pathways of importance in the pathogenesis of systemic lupus erythematosus (SLE) have been identified and clinical trials of biologic agents targeting a number of these are underway
-
The clinical efficacy of belimumab, a human monoclonal antibody that binds B lymphocyte stimulator, has been demonstrated in two phase III studies
-
Monoclonal antibodies against tumor necrosis factor, interferon-α, interleukin-6 and interferon-γ are in early-phase trials, and there are several other potential cytokine targets for SLE therapy
-
Owing to the heterogeneous nature of SLE, the precise cytokine target will probably differ between individual patients and could also vary according to disease stage
-
New and improved biomarkers are needed in order to optimize anticytokine therapy for established disease and also to prevent disease exacerbation
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rahman, A. & Isenberg, D. A. Systemic lupus erythematosus. N. Engl. J. Med. 358, 929–939 (2008).
Manzi, S. et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am. J. Epidemiol. 145, 408–415 (1997).
Graham, R. R., Hom, G., Ortmann, W. & Behrens, T. W. Review of recent genome-wide association scans in lupus. J. Intern. Med. 265, 680–688 (2009).
Moser, K. L., Kelly, J. A., Lessard, C. J. & Harley, J. B. Recent insights into the genetic basis of systemic lupus erythematosus. Genes Immun. 10, 373–379 (2009).
Clarke, A. & Vyse, T. J. Genetics of rheumatic disease. Arthritis Res. Ther. 11, 248 (2009).
Niewold, T. B. et al. Association of the IRF5 risk haplotype with high serum interferon-α activity in systemic lupus erythematosus patients. Arthritis Rheum. 58, 2481–2487 (2008).
Kariuki, S. N. et al. Cutting edge: autoimmune disease risk variant of STAT4 confers increased sensitivity to IFN-α in lupus patients in vivo. J. Immunol. 182, 34–38 (2009).
Vereecke, L., Beyaert, R. & van Loo, G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 30, 383–391 (2009).
Groom, J. R. et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J. Exp. Med. 204, 1959–1971 (2007).
Zhang, J. et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J. Immunol. 166, 6–10 (2001).
Roschke, V. et al. BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J. Immunol. 169, 4314–4321 (2002).
Mackay, F. & Schneider, P. Cracking the BAFF code. Nat. Rev. Immunol. 9, 491–502 (2009).
Castigli, E. et al. TACI and BAFF-R mediate isotype switching in B cells. J. Exp. Med. 201, 35–39 (2005).
Avery, D. T. et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J. Clin. Invest. 112, 286–297 (2003).
Zhang, X. et al. BAFF supports human B cell differentiation in the lymphoid follicles through distinct receptors. Int. Immunol. 17, 779–788 (2005).
Wallace, D. J. et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum. 61, 1168–1178 (2009).
Furie, R. A. et al. Novel evidence-based systemic lupus erythematosus responder index. Arthritis Rheum. 61, 1143–1151 (2009).
Navarra, S. et al. Belimumab, a BLyS-specific inhibitor, reduced disease activity, flares, and prednisone use in patients with active SLE: efficacy and safety results from the phase 3 BLISS-52 study. Presented at the American College of Rheumatology 2009 Annual Scientific Meeting (2009).
GlaxoSmithKline. GlaxoSmithKline and Human Genome Sciences announce positive results in second of two phase 3 trials of Benlysta in systemic lupus erythematosus [online], (2009).
Dall'Era, M. et al. Reduced B lymphocyte and immunoglobulin levels after atacicept treatment in patients with systemic lupus erythematosus: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating trial. Arthritis Rheum. 56, 4142–4150 (2007).
Tak, P. P. et al. Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis Rheum. 58, 61–72 (2008).
Studnicka-Benke, A., Steiner, G., Petera, P. & Smolen, J. S. Tumour necrosis factor alpha and its soluble receptors parallel clinical disease and autoimmune activity in systemic lupus erythematosus. Br. J. Rheumatol. 35, 1067–1074 (1996).
Malide, D., Russo, P. & Bendayan, M. Presence of tumor necrosis factor alpha and interleukin-6 in renal mesangial cells of lupus nephritis patients. Hum. Pathol. 26, 558–564 (1995).
Aringer, M. & Smolen, J. S. The role of tumor necrosis factor-alpha in systemic lupus erythematosus. Arthritis Res. Ther. 10, 202 (2008).
Vassalli, P. The pathophysiology of tumor necrosis factors. Annu. Rev. Immunol. 10, 411–452 (1992).
McDevitt, H., Munson, S., Ettinger, R. & Wu, A. Multiple roles for tumor necrosis factor-α and lymphotoxin α/β in immunity and autoimmunity. Arthritis Res. 4 (Suppl. 3), S141–S152 (2002).
Båve, U., Vallin, H., Alm, G. V. & Rönnblom, L. Activation of natural interferon-α producing cells by apoptotic U937 cells combined with lupus IgG and its regulation by cytokines. J. Autoimmun. 17, 71–80 (2001).
Ramos-Casals, M. et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 86, 242–251 (2007).
Aringer, M. et al. Adverse events and efficacy of TNF-α blockade with infliximab in patients with systemic lupus erythematosus: long-term follow-up of 13 patients. Rheumatology (Oxford) 48, 1451–1454 (2009).
Ytterberg, S. R. & Schnitzer, T. J. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum. 25, 401–406 (1982).
Bengtsson, A. et al. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not antiretroviral antibodies. Lupus 9, 664–671 (2000).
Rönnblom, L. E., Alm, G. V. & Öberg, K. E. Possible induction of systemic lupus erythematosus by interferon-α treatment in a patient with a malignant carcinoid tumour. J. Intern. Med. 227, 207–210 (1990).
Bennett, L. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197, 711–723 (2003).
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003).
Crow, M. K. & Wohlgemuth, J. Microarray analysis of gene expression in lupus. Arthritis Res. Ther. 5, 279–287 (2003).
Peterson, K. S. et al. Characterization of heterogeneity in the molecular pathogenesis of lupus nephritis from transcriptional profiles of laser-captured glomeruli. J. Clin. Invest. 113, 1722–1733 (2004).
Theofilopoulos, A. N., Baccala, R., Beutler, B. & Kono, D. H. Type I interferons (α/β) in immunity and autoimmunity. Ann. Rev. Immunol. 23, 307–336 (2005).
Baccala, R., Hoebe, K., Kono, D. H., Beutler, B. & Theofilopoulos, A. N. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat. Med. 13, 543–551 (2007).
Santiago-Raber, M. L. et al. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J. Exp. Med. 197, 777–788 (2003).
Braun, D., Geraldes, P. & Demengeot, J. Type I interferon controls the onset and severity of autoimmune manifestations in lpr mice. J. Autoimmun. 20, 15–25 (2003).
Wallace, D. J. et al. MEDI-545, an anti-interferon alpha monoclonal antibody, shows evidence of clinical activity in systemic lupus erythematosus [abstract 1315]. Arthritis Rheum. 56 (Suppl.), S526–S527 (2007).
Yao, Y. et al. Neutralization of interferon-α/β-inducible genes and downstream effect in a phase I trial of an anti-interferon-α monoclonal antibody in systemic lupus erythematosus. Arthritis Rheum. 60, 1785–1796 (2009).
McBride, J. M. et al. Dose-dependent modulation of interferon regulated genes with administration of single and repeat doses of rontalizumab in a phase I, placebo controlled, double blind, dose escalation study in SLE [abstract 2072]. Arthritis Rheum. 60 (Suppl.), S775–S776 (2009).
Kishimoto, T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res. Ther. 8 (Suppl. 2), S2 (2006).
Smolen, J. S. et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 371, 987–997 (2008).
Illei, G. G. et al. Tocilizumab in systemic lupus erythematosus: Data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 62, 542–552 (2010).
al-Janadi, M., al-Balla, S., al-Dalaan, A. & Raziuddin, S. Cytokine profile in systemic lupus erythematosus, rheumatoid arthritis, and other rheumatic diseases. J. Clin. Immunol. 13, 58–67 (1993).
Akahoshi, M. et al. Th1/Th2 balance of peripheral T helper cells in systemic lupus erythematosus. Arthritis Rheum. 42, 1644–1648 (1999).
Uhm, W. S. et al. Cytokine balance in kidney tissue from lupus nephritis patients. Rheumatology (Oxford) 42, 935–938 (2003).
Min, D. J. et al. Decreased production of interleukin-12 and interferon-gamma is associated with renal involvement in systemic lupus erythematosus. Scand. J. Rheumatol. 30, 159–163 (2001).
Kirou, K. A. et al. Coordinate overexpression of interferon-α-induced genes in systemic lupus erythematosus. Arthritis Rheum. 50, 3958–3967 (2004).
Jacob, C. O., van der Meide, P. H. & McDevitt, H. O. In vivo treatment of (NZB × NZW)F1 lupus-like nephritis with monoclonal antibody to gamma interferon. J. Exp. Med. 166, 798–803 (1987).
Haas, C., Ryffel, B. & Le Hir, M. IFN-γ receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone (NZB × NZW)F1 mice. J. Immunol. 160, 3713–3718 (1998).
Hu, X. & Ivashkiv, L. B. Cross-regulation of signaling pathways by interferon-γ: implications for immune responses and autoimmune diseases. Immunity 31, 539–550 (2009).
Schroder, K., Hertzog, P. J., Ravasi, T. & Hume, D. A. Interferon-γ: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75, 163–189 (2004).
Harigai, M. et al. Excessive production of IFN-γ in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B. J. Immunol. 181, 2211–2219 (2008).
Dinarello, C. A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Sturfelt, G., Roux-Lombard, P., Wollheim, F. A. & Dayer, J. M. Low levels of interleukin-1 receptor antagonist coincide with kidney involvement in systemic lupus erythematosus. Br. J. Rheumatol. 36, 1283–1289 (1997).
Tucci, M. et al. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum. 58, 251–262 (2008).
Ostendorf, B. et al. Preliminary results of safety and efficacy of the interleukin 1 receptor antagonist anakinra in patients with severe lupus arthritis. Ann. Rheum. Dis. 64, 630–633 (2005).
Moosig, F., Zeuner, R., Renk, C. & Schroder, J. O. IL-1RA in refractory systemic lupus erythematosus. Lupus 13, 605–606 (2004).
Park, Y. B. et al. Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clin. Exp. Rheumatol. 16, 283–288 (1998).
Llorente, L. et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J. Exp. Med. 181, 839–844 (1995).
Mosser, D. M. & Zhang, X. Interleukin-10: new perspectives on an old cytokine. Immunol. Rev. 226, 205–218 (2008).
Llorente, L. et al. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 43, 1790–1800 (2000).
Yang, J. et al. TH17 and natural TREG cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 60, 1472–1483 (2009).
Ouyang, W., Kolls, J. K. & Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28, 454–467 (2008).
Wong, C. K. et al. Elevated production of B cell chemokine CXCL13 is correlated with systemic lupus erythematosus disease activity. J. Clin. Immunol. 30, 45–52 (2010).
Sawalha, A. H. et al. Genetic association of interleukin-21 polymorphisms with systemic lupus erythematosus. Ann. Rheum. Dis. 67, 458–461 (2008).
Vinuesa, C. G. et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435, 452–458 (2005).
Bubier, J. A. et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc. Natl Acad. Sci. USA 106, 1518–1523 (2009).
Rochman, Y., Spolski, R. & Leonard, W. J. New insights into the regulation of T cells by γc family cytokines. Nat. Rev. Immunol. 9, 480–490 (2009).
Strengell, M., Julkunen, I. & Matikainen, S. IFN-α regulates IL-21 and IL-21R expression in human NK and T cells. J. Leukoc. Biol. 76, 416–422 (2004).
Bertsias, G., Gordon, C. & Boumpas, D. T. Clinical trials in systemic lupus erythematosus (SLE): lessons from the past as we proceed to the future—the EULAR recommendations for the management of SLE and the use of end-points in clinical trials. Lupus 17, 437–442 (2008).
Gordon, C. et al. EULAR points to consider for conducting clinical trials in systemic lupus erythematosus. Ann. Rheum. Dis. 68, 470–476 (2009).
Zagury, D. et al. IFNα kinoid vaccine-induced neutralizing antibodies prevent clinical manifestations in a lupus flare murine model. Proc. Natl Acad. Sci. USA 106, 5294–5299 (2009).
Hu, N., Long, H., Zhao, M., Yin, H. & Lu, Q. Aberrant expression pattern of histone acetylation modifiers and mitigation of lupus by SIRT1-siRNA in MRL/lpr mice. Scand. J. Rheumatol. 38, 464–471 (2009).
St Clair, E. W. Novel targeted therapies for autoimmunity. Curr. Opin. Immunol. 21, 648–657 (2009).
Sigurdsson, S. et al. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am. J. Hum. Gen. 76, 528–537 (2005).
Sigurdsson, S. et al. A risk haplotype of STAT4 for systemic lupus erythematosus is over-expressed, correlates with anti-dsDNA and shows additive effects with two risk alleles of IRF5. Hum. Mol. Genet. 17, 2868–2876 (2008).
Jacob, C. O. et al. Identification of IRAK1 as a risk gene with critical role in the pathogenesis of systemic lupus erythematosus. Proc. Natl Acad. Sci. USA 106, 6256–6261 (2009).
Graham, R. R. et al. Genetic variants near TNFAIP3 on 6q23 are associated with systemic lupus erythematosus. Nat. Genet. 40, 1059–1061 (2008).
Harley, J. B. et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat. Genet. 40, 204–210 (2008).
Gateva, V. et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat. Genet. 41, 1228–1233 (2009).
Kim, K. et al. Interferon-gamma gene polymorphisms associated with susceptibility to systemic lupus erythematosus. Ann. Rheum. Dis. doi:10.1136/ard.2009.117572.
Webb, R. et al. A polymorphism within IL21R confers risk for systemic lupus erythematosus. Arthritis Rheum. 60, 2402–2407 (2009).
Acknowledgements
This work was supported by The Alliance for Lupus Research, the Swedish Research Council, the Dana Foundation, the Swedish Rheumatism Association, the King Gustaf V 80th Birthday Foundation, the Söderbergs Foundation, COMBINE, the Leap for Lupus Foundation and the NIH. The authors thank Jeffrey Ledbetter and Gunnar Alm for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
L. Ronnblom has acted as a consultant for Active Biotech Research Inc., Medimmune Inc., Neovacs and Novo Nordisk A/S, and has and has participated in a clinical trial sponsored by Active Biotech. K. B. Elkon has received research grants from Hoffman La Roche, Merck Serono International S.A. (an affiliate of Merck KGaA, Darmstadt, Germany) and Zymogenetics.
Rights and permissions
About this article
Cite this article
Rönnblom, L., Elkon, K. Cytokines as therapeutic targets in SLE. Nat Rev Rheumatol 6, 339–347 (2010). https://doi.org/10.1038/nrrheum.2010.64
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2010.64
This article is cited by
-
Enteric Toll-like receptor 7 stimulation causes acute exacerbation in lupus-susceptible mice
Clinical Rheumatology (2023)
-
JAK inhibitors: a potential treatment for JDM in the context of the role of interferon-driven pathology
Pediatric Rheumatology (2021)
-
Neuropsychiatric lupus: new mechanistic insights and future treatment directions
Nature Reviews Rheumatology (2019)
-
JAK3-STAT pathway blocking benefits in experimental lupus nephritis
Arthritis Research & Therapy (2016)
-
Anti-dsDNA antibodies bind to TLR4 and activate NLRP3 inflammasome in lupus monocytes/macrophages
Journal of Translational Medicine (2016)