Abstract
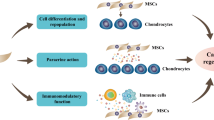
Mesenchymal stem cells (MSCs), or multipotent mesenchymal stromal cells as they are also known, have been identified in bone marrow as well as in other tissues of the joint, including adipose, synovium, periosteum, perichondrium, and cartilage. These cells are characterized by their phenotype and their ability to differentiate into three lineages: chondrocytes, osteoblasts and adipocytes. Importantly, MSCs also potently modulate immune responses, exhibit healing capacities, improve angiogenesis and prevent fibrosis. These properties might be explained at least in part by the trophic effects of MSCs through the secretion of a number of cytokines and growth factors. However, the mechanisms involved in the differentiation potential of MSCs, and their immunomodulatory and paracrine properties, are currently being extensively studied. These unique properties of MSCs confer on them the potential to be used for therapeutic applications in rheumatic diseases, including rheumatoid arthritis, osteoarthritis, genetic bone and cartilage disorders as well as bone metastasis.
Key Points
-
In addition to their differentiation potential, mesenchymal stem cells (MSCs) exhibit immunosuppressive activity, as shown by their ability to inhibit the proliferation and function of immune cells
-
The immunosuppressive effect of MSCs is mediated by soluble factors
-
Another important feature of MSCs is their capacity to stimulate resident cells or to attract cells via a paracrine mode of action
-
The usefulness of MSCs for bone and cartilage repair has been well documented in various experimental models
-
The therapeutic benefit of MSCs for osteoarthritis, osteogenesis imperfecta and graft-versus-host disease is under evaluation in humans
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Djouad, F., Mrugala, D., Noël, D. & Jorgensen, C. Engineered mesenchymal stem cells for cartilage repair. Regen. Med. 1, 529–537 (2006).
Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 (2006).
Pittenger, M., Vanguri, P., Simonetti, D. & Young, R. Adult mesenchymal stem cells: potential for muscle and tendon regeneration and use in gene therapy. J. Musculoskelet. Neuronal Interact. 2, 309–320 (2002).
Makino, S. et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J. Clin. Invest. 103, 697–705 (1999).
Tropel, P. et al. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells 24, 2868–2876 (2006).
Bhatia, R. & Hare, J. M. Mesenchymal stem cells: future source for reparative medicine. Congest. Heart Fail. 11, 87–91 (2005).
Vinatier, C., Mrugala, D., Jorgensen, C., Guicheux, J. & Noël, D. Cartilage engineering: a critical combination of cells, biomaterials and biofactors. Trends Biotech. 27, 307–314 (2009).
Alsalameh, S., Amin, R., Gemba, T. & Lotz, M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 50, 1522–1532 (2004).
Djouad, F. et al. Transcriptional profiles discriminate bone marrow-derived and synovium-derived mesenchymal stem cells. Arthritis Res. Ther. 7, R1304–R1315 (2005).
De Bari, C., Dell'Accio, F. & Luyten, F. P. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 44, 85–95 (2001).
Gimble, J. M., Katz, A. J. & Bunnell, B. A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 100, 1249–1260 (2007).
Fritz, V. et al. Antitumoral activity and osteogenic potential of mesenchymal stem cells expressing the urokinase-type plasminogen antagonist amino-terminal fragment in a murine model of osteolytic tumor. Stem Cells 26, 2981–2990 (2008).
Planat-Benard, V. et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 109, 656–663 (2004).
Mazo, M. et al. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur. J. Heart Fail. 10, 454–462 (2008).
Rehman, J. et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109, 1292–1298 (2004).
Croft, A. P. & Przyborski, S. A. Formation of neurons by non-neural adult stem cells: potential mechanism implicates an artifact of growth in culture. Stem Cells 24, 1841–1851 (2006).
Hiraoka, K., Grogan, S., Olee, T. & Lotz, M. Mesenchymal progenitor cells in adult human articular cartilage. Biorheology 43, 447–454 (2006).
Hattori, S., Oxford, C. & Reddi, A. H. Identification of superficial zone articular chondrocyte stem/progenitor cells. Biochem. Biophys. Res. Commun. 358, 99–103 (2007).
Fickert, S., Fiedler, J. & Brenner, R. E. Identification, quantification and isolation of mesenchymal progenitor cells from osteoarthritic synovium by fluorescence automated cell sorting. Osteoarthritis Cartilage 11, 790–800 (2003).
Jones, S., Horwood, N., Cope, A. & Dazzi, F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J. Immunol. 179, 2824–2831 (2007).
Nemeth, K. et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 15, 42–49 (2009).
Groh, M. E., Maitra, B., Szekely, E. & Koc, O. N. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp. Hematol. 33, 928–934 (2005).
Le Blanc, K., Tammik, L., Sundberg, B., Haynesworth, S. E. & Ringden, O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand. J. Immunol. 57, 11–20 (2003).
Ren, G. et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150 (2008).
Spaggiari, G. M. et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2, 3-dioxygenase and prostaglandin E2. Blood 111, 1327–1333 (2008).
Noël, D., Djouad, F., Bouffi, C., Mrugala, D. & Jorgensen, C. Multipotent mesenchymal stromal cells and immune tolerance. Leuk. Lymphoma 48, 1283–1289 (2007).
Gieseke, F. et al. Human multipotent mesenchymal stromal cells inhibit proliferation of PBMCs independently of IFNgammaR1 signaling and IDO expression. Blood 110, 2197–2200 (2007).
Djouad, F. et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 25, 2025–2032 (2007).
Sato, K. et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 109, 228–234 (2007).
Aggarwal, S. & Pittenger, M. F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822 (2005).
Jarvinen, L. et al. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J. Immunol. 181, 4389–4396 (2008).
Rasmusson, I. Immune modulation by mesenchymal stem cells. Exp. Cell Res. 312, 2169–2179 (2006).
Chabannes, D. et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood 110, 3691–3694 (2007).
Nasef, A. et al. Leukemia inhibitory factor: role in human mesenchymal stem cells mediated immunosuppression. Cell. Immunol. 253, 16–22 (2008).
Selmani, Z. et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 26, 212–222 (2008).
Djouad, F. et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 102, 3837–3844 (2003).
Augello, A., Tasso, R., Negrini, S. M., Cancedda, R. & Pennesi, G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 56, 1175–1186 (2007).
Bartholomew, A. et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 30, 42–48 (2002).
Glennie, S., Soeiro, I., Dyson, P. J., Lam, E. W. & Dazzi, F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 105, 2821–2827 (2005).
Zappia, E. et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 106, 1755–1761 (2005).
Rasmusson, I., Ringden, O., Sundberg, B. & Le Blanc, K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 76, 1208–1213 (2003).
Karlsson, H. et al. Mesenchymal stem cells exert differential effects on alloantigen and virus-specific T-cell responses. Blood 112, 532–541 (2008).
Maccario, R. et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 90, 516–525 (2005).
Prevosto, C., Zancolli, M., Canevali, P., Zocchi, M. R. & Poggi, A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica 92, 881–888 (2007).
Jiang, X. X. et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 105, 4120–4126 (2005).
Nauta, A. J., Kruisselbrink, A. B., Lurvink, E., Willemze, R. & Fibbe, W. E. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J. Immunol. 177, 2080–2087 (2006).
Spaggiari, G. M., Capobianco, A., Becchetti, S., Mingari, M. C. & Moretta, L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 107, 1484–1490 (2006).
Sotiropoulou, P. A., Perez, S. A., Gritzapis, A. D., Baxevanis, C. N. & Papamichail, M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 24, 74–85 (2006).
Corcione, A. et al. Human mesenchymal stem cells modulate B-cell functions. Blood 107, 367–372 (2006).
Rasmusson, I., Le Blanc, K., Sundberg, B. & Ringden, O. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand. J. Immunol. 65, 336–343 (2007).
Raffaghello, L. et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells 26, 151–162 (2008).
Boumaza, I. et al. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J. Autoimmun. 32, 33–44 (2009).
Casiraghi, F. et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J. Immunol. 181, 3933–3946 (2008).
Caplan, A. I. & Dennis, J. E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 98, 1076–1084 (2006).
Ringden, O. & Le Blanc, K. Allogeneic hematopoietic stem cell transplantation: state of the art and new perspectives. APMIS 113, 813–830 (2005).
Takahashi, N., Udagawa, N. & Suda, T. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem. Biophys. Res. Commun. 256, 449–455 (1999).
Tang, Y. L. et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul. Pept. 117, 3–10 (2004).
Nagaya, N. et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am. J. Physiol. Heart Circ. Physiol. 287, H2670–H2676 (2004).
Li, Y. et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia 49, 407–417 (2005).
Wynn, T. A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 (2008).
Li, L. et al. Mesenchymal stem cell transplantation attenuates cardiac fibrosis associated with isoproterenol-induced global heart failure. Transpl. Int. 21, 1181–1189 (2008).
Ohnishi, S., Sumiyoshi, H., Kitamura, S. & Nagaya, N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Lett. 581, 3961–3966 (2007).
Lane, J. M. et al. Bone marrow and recombinant human bone morphogenetic protein-2 in osseous repair. Clin. Orthop. Relat. Res. 361, 216–227 (1999).
Hou, R. et al. Comparative study between coral–mesenchymal stem cells–rhBMP-2 composite and auto-bone-graft in rabbit critical-sized cranial defect model. J. Biomed. Mater. Res. A 80, 85–93 (2007).
Hernigou, P. et al. Percutaneous autologous bone-marrow grafting for nonunions. Surgical technique. J. Bone Joint Surg. Am. 88 (Suppl. 1 Pt 2), 322–327 (2006).
Chow, J. C., Hantes, M. E., Houle, J. B. & Zalavras, C. G. Arthroscopic autogenous osteochondral transplantation for treating knee cartilage defects: a 2- to 5-year follow-up study. Arthroscopy 20, 681–690 (2004).
Chu, C. R., Convery, F. R., Akeson, W. H., Meyers, M. & Amiel, D. Articular cartilage transplantation. Clinical results in the knee. Clin. Orthop. Relat. Dis. 360, 159–168 (1999).
Stone, K. R., Walgenbach, A. W., Freyer, A., Turek, T. J. & Speer, D. P. Articular cartilage paste grafting to full-thickness articular cartilage knee joint lesions: a 2- to 12-year follow-up. Arthroscopy 22, 291–299 (2006).
Mrugala, D. et al. Phenotypic and functional characterisation of ovine mesenchymal stem cells: application to a cartilage defect model. Ann. Rheum. Dis. 67, 288–295 (2008).
Li, W. J. et al. Evaluation of articular cartilage repair using biodegradable nanofibrous scaffolds in a swine model: a pilot study. J. Tissue Eng. Regen. Med. 3, 1–10 (2009).
Murphy, J. M., Fink, D. J., Hunziker, E. B. & Barry, F. P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 48, 3464–3474 (2003).
Le Blanc, K. et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation 79, 1607–1614 (2005).
Horwitz, E. M. et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 5, 309–313 (1999).
Ringden, O. et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 81, 1390–1397 (2006).
Lee, R. H. et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc. Natl Acad. Sci. USA 103, 17438–17443 (2006).
Djouad, F. et al. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 52, 1595–1603 (2005).
Zheng, Z. H., Li, X. Y., Ding, J., Jia, J. F. & Zhu, P. Allogeneic mesenchymal stem cell and mesenchymal stem cell-differentiated chondrocyte suppress the responses of type II collagen-reactive T cells in rheumatoid arthritis. Rheumatology (Oxford) 47, 22–30 (2008).
Studeny, M. et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J. Natl Cancer Inst. 96, 1593–1603 (2004).
Chen, X. C. et al. Prophylaxis against carcinogenesis in three kinds of unestablished tumor models via IL12-gene-engineered MSCs. Carcinogenesis 27, 2434–2441 (2006).
Mishra, P. J., Mishra, P. J., Glod, J. W. & Banerjee, D. Mesenchymal stem cells: flip side of the coin. Cancer Res. 69, 1255–1258 (2009).
Noël, D. et al. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp. Cell Res. 314, 1575–1584 (2008).
Di Cesare, P. E., Frenkel, S. R., Carlson, C. S., Fang, C. & Liu, C. Regional gene therapy for full-thickness articular cartilage lesions using naked DNA with a collagen matrix. J. Orthop. Res. 24, 1118–1127 (2006).
Kuo, A. C. et al. Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthritis Cartilage 14, 1126–1135 (2006).
Kuroda, R. et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 54, 433–442 (2006).
Garrison, K. R. et al. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technol. Assess. 11, 1–150, iii–iv (2007).
Acknowledgements
D. Noël and C. Jorgensen contributed equally to this article. This work is supported by the Inserm Institute, the University of Montpellier I, The French National Research Agency, Fondation de l'Avenir pour la Recherche Médicale Appliquée and the Fondation pour la Recherche Médicale. The authors thank Jean-Louis Pasquier for the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Djouad, F., Bouffi, C., Ghannam, S. et al. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol 5, 392–399 (2009). https://doi.org/10.1038/nrrheum.2009.104
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2009.104
This article is cited by
-
PLOD2, a key factor for MRL MSC metabolism and chondroprotective properties
Stem Cell Research & Therapy (2024)
-
Transfer of mesenchymal stem cell mitochondria to CD4+ T cells contributes to repress Th1 differentiation by downregulating T-bet expression
Stem Cell Research & Therapy (2023)
-
A novel visceral adiposity index predicts bone loss in female early rheumatoid arthritis patients detected by HR-pQCT
Scientific Reports (2023)
-
Anti-inflammatory and anti-oxidant activities of mesenchymal stem cells in chemically induced arthritic rats
Molecular Biology Reports (2023)
-
Injection laryngoplasty of human adipose-derived stem cell spheroids with hyaluronic acid-based hydrogel improves the morphological and functional characteristics of geriatric larynx
Biomaterials Research (2022)